Curvature-Influenced Electrocatalytic NRR Reactivity by Heme-like FeN4-Site on Carbon Materials
Abstract
1. Introduction
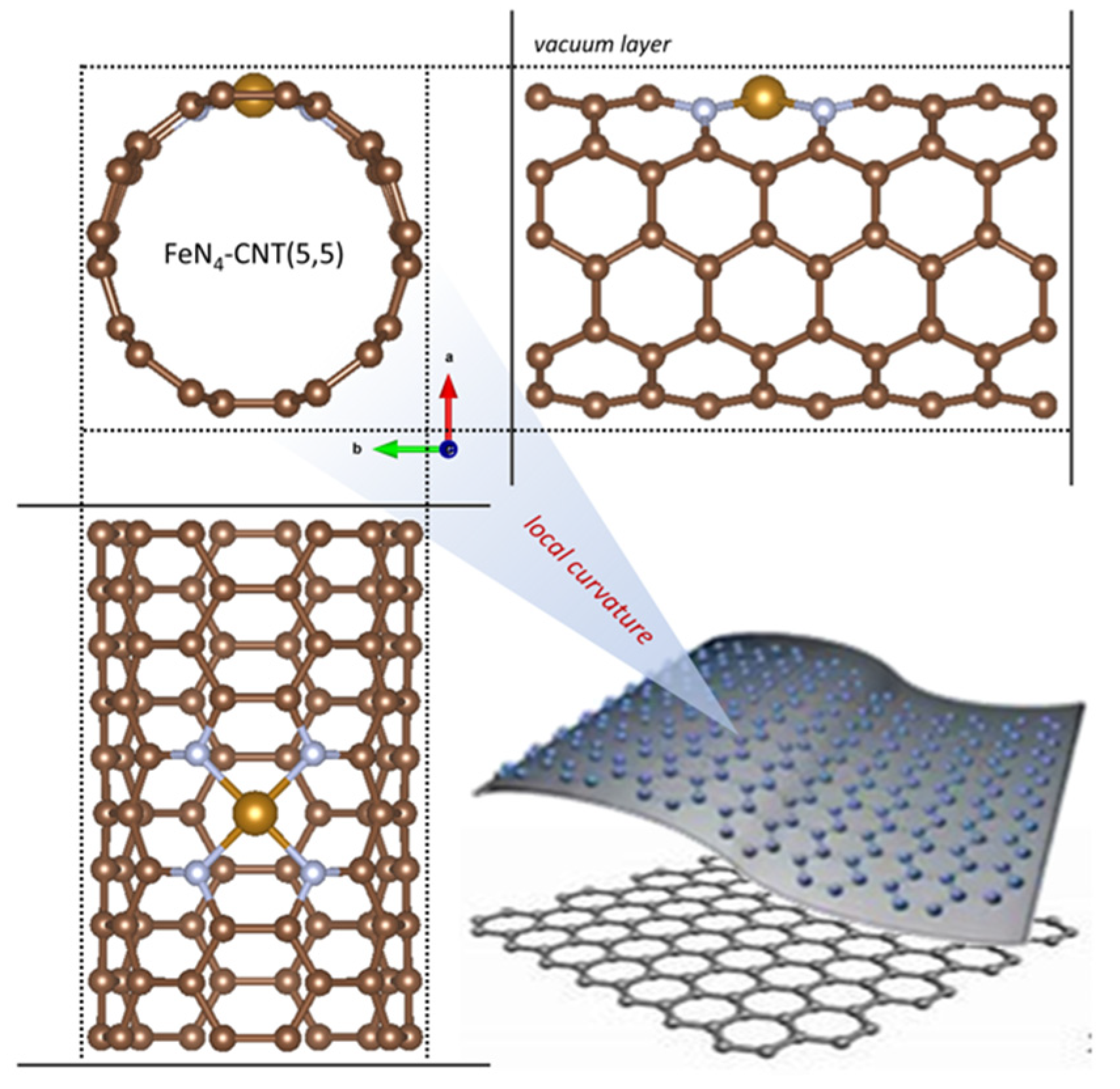
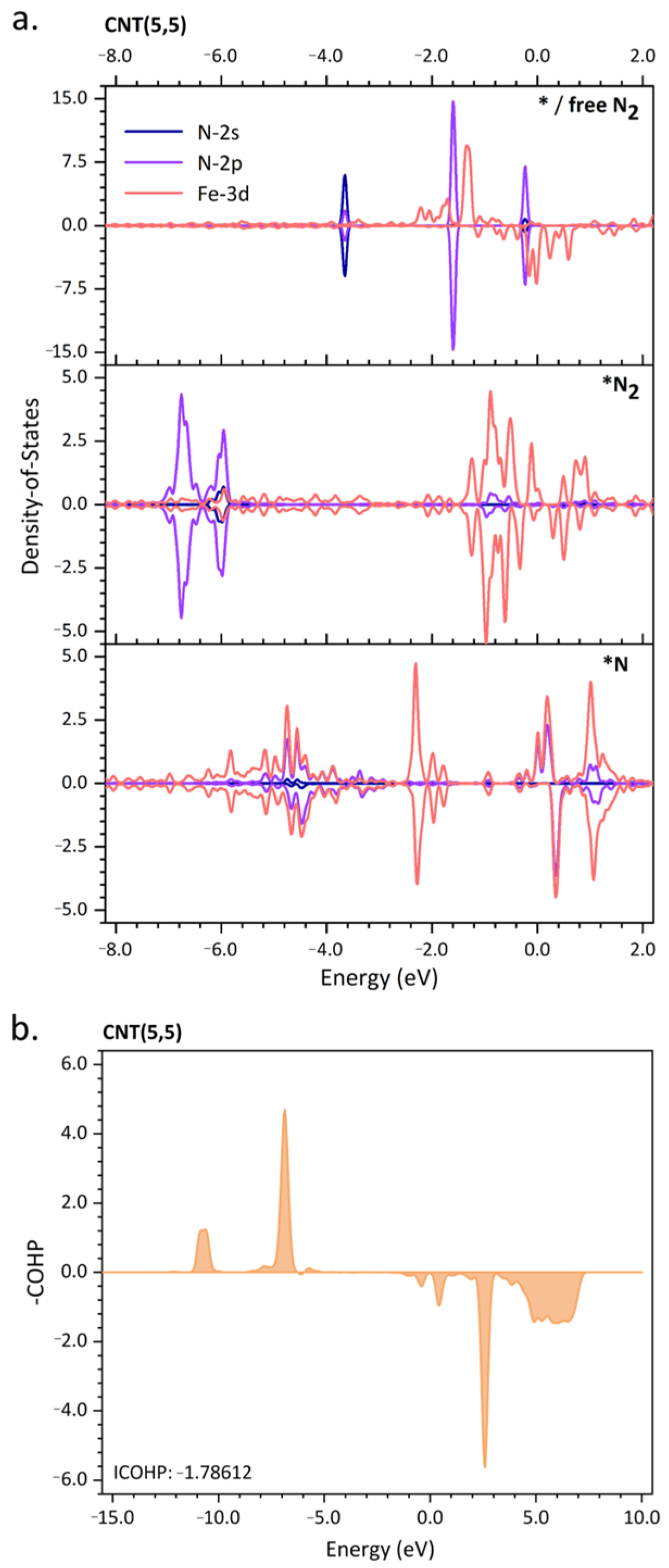
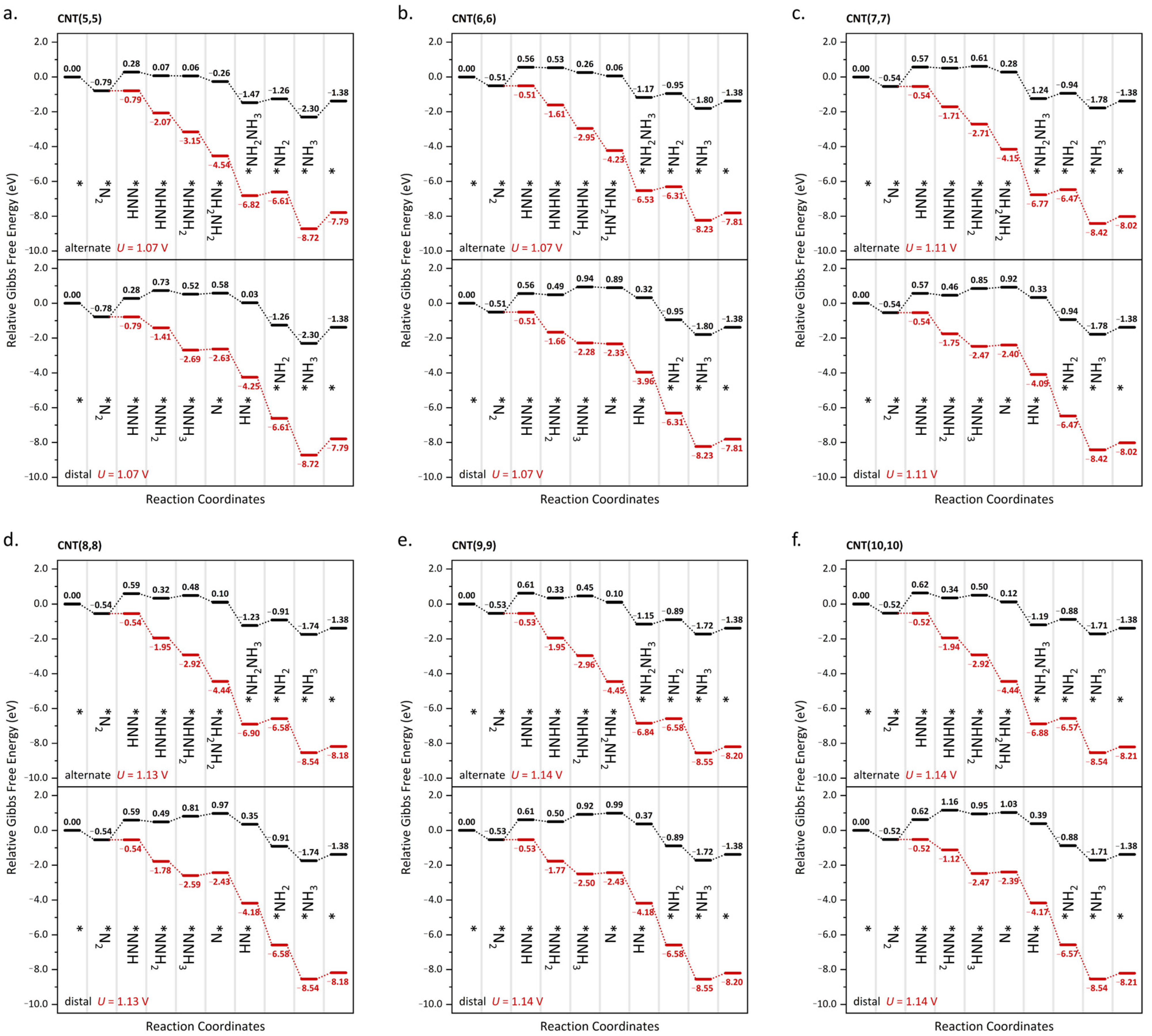
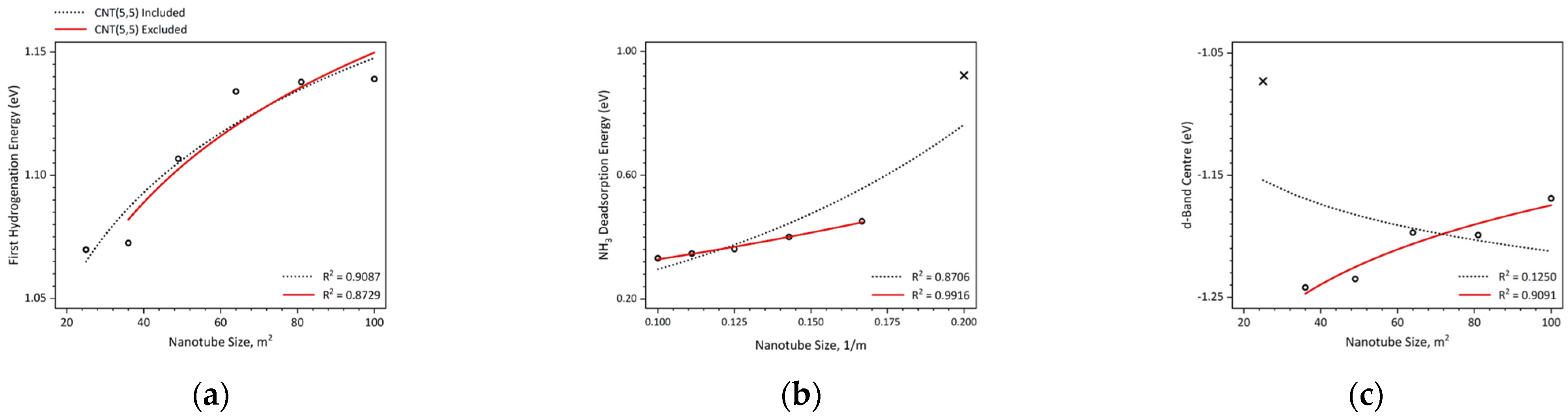
2. Results and Discussion
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Q.; Shen, Y.; Chen, B. Synthesis, decoration and properties of three-dimensional graphene-based macrostructures: A review. Chem. Eng. J. 2015, 264, 753–771. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, Z.; Wu, X.; Lv, Z.; Wu, P.; Cai, C. Graphdiyne-supported single-atom-sized Fe catalysts for the oxygen reduction reaction: DFT predictions and experimental validations. ACS Catal. 2018, 8, 10364–10374. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Li, H.; He, S.; Veder, J.-P.; Johannessen, B.; Xiao, J.; Lu, S.; Pan, J.; Chisholm, M.F. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2. Appl. Catal. B Environ. 2019, 243, 294–303. [Google Scholar] [CrossRef]
- Mitchell, S.; Pérez-Ramírez, J. Single atom catalysis: A decade of stunning progress and the promise for a bright future. Nat. Commun. 2020, 11, 4302. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-atom catalysts: From design to application. Electrochem. Energy Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef]
- Katiyar, A.K.; Hoang, A.T.; Xu, D.; Hong, J.; Kim, B.J.; Ji, S.; Ahn, J.-H. 2D Materials in Flexible Electronics: Recent Advances and Future Prospectives. Chem. Rev. 2024, 124, 318–419. [Google Scholar] [CrossRef]
- Yu, W.; Gong, K.; Li, Y.; Ding, B.; Li, L.; Xu, Y.; Wang, R.; Li, L.; Zhang, G.; Lin, S. Flexible 2D Materials beyond Graphene: Synthesis, Properties, and Applications. Small 2022, 18, 2105383. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Feng, Y.; Allen, C.S.; Wan, C.; Volosskiy, B.; Li, M.; Zhao, Z.; Wang, Y.; Sun, H. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Zhong, W.; Yue, J.; Zhang, R.; Huang, H.; Huang, H.; Shen, Z.; Jiang, L.; Xu, M.; Xia, Q.; Cao, Y. Screening of Transition Metal Supported on Black Phosphorus as Electrocatalysts for CO2 Reduction. Inorg. Chem. 2024, 63, 1035–1045. [Google Scholar]
- Xia, J.; Xu, J.; Yu, B.; Liang, X.; Qiu, Z.; Li, H.; Feng, H.; Li, Y.; Cai, Y.; Wei, H.; et al. A Metal–Sulfur–Carbon Catalyst Mimicking the Two-Component Architecture of Nitrogenase. Angew. Chem. Int. Ed. 2024, 63, e202412740. [Google Scholar]
- You, X.; Xu, J.; Zhuang, Z.; Xia, J.; Wang, S.; Wei, H.; Li, Y.; Cai, Y.; Xiang, H.; Yu, B. Ru-Ni alloy nanosheets as tandem catalysts for electrochemical reduction of nitrate to ammonia. Nano Res. 2024, 17, 4815–4824. [Google Scholar]
- Liang, Z.; Kong, N.; Yang, C.; Zhang, W.; Zheng, H.; Lin, H.; Cao, R. Highly curved nanostructure-coated Co, N-doped carbon materials for oxygen electrocatalysis. Angew. Chem. 2021, 133, 12869–12874. [Google Scholar]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576–591. [Google Scholar]
- Wang, Z.; Gao, S.; Liu, X.; Chen, X.; Zhang, X.; Sa, R.; Li, Q.; Sun, C.; Ma, Z. Defective SiC nanotube based single-atom catalysts for electrocatalytic nitrogen fixation with curvature effect. Mol. Catal. 2023, 549, 113519. [Google Scholar]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef]
- Yan, X.; Duan, C.; Yu, S.; Dai, B.; Sun, C.; Chu, H. Recent advances on CO2 reduction reactions using single-atom catalysts. Renew. Sustain. Energy Rev. 2024, 190, 114086. [Google Scholar]
- Shi, H.; He, Y.; Li, Y.; He, T.; Luo, P. Efficient degradation of tetracycline in real water systems by metal-free g-C3N4 microsphere through visible-light catalysis and PMS activation synergy. Sep. Purif. Technol. 2022, 280, 119864. [Google Scholar]
- Wang, Y.; Bao, Z.; Shi, M.; Liang, Z.; Cao, R.; Zheng, H. The role of surface curvature in electrocatalysts. Chem. A Eur. J. 2022, 28, e202102915. [Google Scholar]
- Mohamed, A.G.A.; Huang, Y.; Xie, J.; Borse, R.A.; Parameswaram, G.; Wang, Y. Metal-free sites with multidimensional structure modifications for selective electrochemical CO2 reduction. Nano Today 2020, 33, 100891. [Google Scholar] [CrossRef]
- Wen, H.; Zhao, Z.; Luo, Z.; Wang, C. Unraveling the Impact of Curvature on Electrocatalytic Performance of Carbon Materials: A State-of-the-Art Review. ChemSusChem 2024, 17, e202301859. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Gu, C.; Chen, J.; Zhu, Y.; Wang, L. Curved Structure Regulated Single Metal Sites for Advanced Electrocatalytic Reactions. Small 2024, 20, 2404758. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, H.; Low, J.; Gao, C.; Long, R.; Xiong, Y. Recent progress on electrocatalyst and photocatalyst design for nitrogen reduction. Small Methods 2019, 3, 1800388. [Google Scholar] [CrossRef]
- Guo, C.; Ran, J.; Vasileff, A.; Qiao, S.-Z. Rational design of electrocatalysts and photo (electro) catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 2018, 11, 45–56. [Google Scholar] [CrossRef]
- Bao, D.; Zhang, Q.; Meng, F.-L.; Zhong, H.-X.; Shi, M.-M.; Zhang, Y.; Yan, J.-M.; Jiang, Q.; Zhang, X.-B. Electrochemical Reduction of N2 under Ambient Conditions for Artificial N2 Fixation and Renewable Energy Storage Using N2/NH3 Cycle. Adv. Mater. 2017, 29, 1604799. [Google Scholar] [CrossRef]
- Zhao, Z.; Long, Y.; Luo, S.; Luo, Y.; Chen, M.; Ma, J. Metal-Free C3N4 with plentiful nitrogen vacancy and increased specific surface area for electrocatalytic nitrogen reduction. J. Energy Chem. 2021, 60, 546–555. [Google Scholar] [CrossRef]
- Guo, P.; Yin, F.; Ni, Z.; Shi, L.; Kofie, G. Efficient Bi catalysts for electrochemical synthesis of ammonia in neutral electrolyte: The effect of Bi particle size on the NRR reaction mechanism. Chem. Eng. J. 2025, 503, 158390. [Google Scholar] [CrossRef]
- Liu, P.; Fu, C.; Li, Y.; Wei, H. Theoretical screening of single atoms anchored on defective graphene for electrocatalytic N2 reduction reactions: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 9322–9329. [Google Scholar] [CrossRef]
- Fu, C.; Li, Y.; Wei, H. Double boron atom-doped graphdiynes as efficient metal-free electrocatalysts for nitrogen reduction into ammonia: A first-principles study. Phys. Chem. Chem. Phys. 2021, 23, 17683–17692. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-doped graphene for electrocatalytic N2 reduction. Joule 2018, 2, 1610–1622. [Google Scholar]
- He, C.; Wu, Z.-Y.; Zhao, L.; Ming, M.; Zhang, Y.; Yi, Y.; Hu, J.-S. Identification of FeN4 as an efficient active site for electrochemical N2 reduction. ACS Catal. 2019, 9, 7311–7317. [Google Scholar]
- Chu, K.; Gu, W.; Li, Q.; Liu, Y.; Tian, Y.; Liu, W. Amorphization activated FeB2 porous nanosheets enable efficient electrocatalytic N2 fixation. J. Energy Chem. 2021, 53, 82–89. [Google Scholar]
- Zhang, L.; Liang, P.; Man, X.-l.; Wang, D.; Huang, J.; Shu, H.-b.; Liu, Z.-g.; Wang, L. Fe, N co-doped graphene as a multi-functional anchor material for lithium-sulfur battery. J. Phys. Chem. Solids 2019, 126, 280–286. [Google Scholar]
- Dronskowski, R.; Blöchl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Qiu, C.; Gan, L.; Ding, J.; Li, F.; Wang, T.; Liu, Y.; Wang, Y.; Tao, H. Boosting activity of Fe-N4 sites in single-Fe-atom catalysts via S in the second coordination sphere for direct methanol fuel cells. Cell Rep. Phys. Sci. 2023, 4, 101330. [Google Scholar]
- Sui, H.; Guo, Q.; Xiang, M.; Kong, X.; Zhang, J.; Ding, S.; Su, Y. Theoretical Insights of Curvature Effects of FeN4-Doped Carbon Nanotubes on ORR Activity. J. Phys. Chem. Lett. 2024, 15, 8257–8264. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]
- Froyen, S. Brillouin-zone integration by Fourier quadrature: Special points for superlattice and supercell calculations. Phys. Rev. B 1989, 39, 3168–3172. [Google Scholar]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [PubMed]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar]
- Takigawa, I.; Shimizu, K.-i.; Tsuda, K.; Takakusagi, S. Machine-learning prediction of the d-band center for metals and bimetals. RSC Adv. 2016, 6, 52587–52595. [Google Scholar]
- Guo, H.; Li, L.; Wang, X.; Yao, G.; Yu, H.; Tian, Z.; Li, B.; Chen, L. Theoretical investigation on the single transition-metal atom-decorated defective MoS2 for electrocatalytic ammonia synthesis. ACS Appl. Mater. Interfaces 2019, 11, 36506–36514. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Huang, Z.; Chen, X.; Li, Y.; Yan, X.; Xu, J.; Wei, H. Curvature-Influenced Electrocatalytic NRR Reactivity by Heme-like FeN4-Site on Carbon Materials. Molecules 2025, 30, 1670. https://doi.org/10.3390/molecules30081670
Meng Y, Huang Z, Chen X, Li Y, Yan X, Xu J, Wei H. Curvature-Influenced Electrocatalytic NRR Reactivity by Heme-like FeN4-Site on Carbon Materials. Molecules. 2025; 30(8):1670. https://doi.org/10.3390/molecules30081670
Chicago/Turabian StyleMeng, Yajie, Ziyue Huang, Xi Chen, Yingqi Li, Xueyuan Yan, Jiawei Xu, and Haiyan Wei. 2025. "Curvature-Influenced Electrocatalytic NRR Reactivity by Heme-like FeN4-Site on Carbon Materials" Molecules 30, no. 8: 1670. https://doi.org/10.3390/molecules30081670
APA StyleMeng, Y., Huang, Z., Chen, X., Li, Y., Yan, X., Xu, J., & Wei, H. (2025). Curvature-Influenced Electrocatalytic NRR Reactivity by Heme-like FeN4-Site on Carbon Materials. Molecules, 30(8), 1670. https://doi.org/10.3390/molecules30081670





