Synthesis of Straw-Based Hydrothermal Carbonation Carbon and Its Photocatalytic Removal of Cr(VI) and Microcystin-LR
Abstract
1. Introduction
2. Results and Discussion
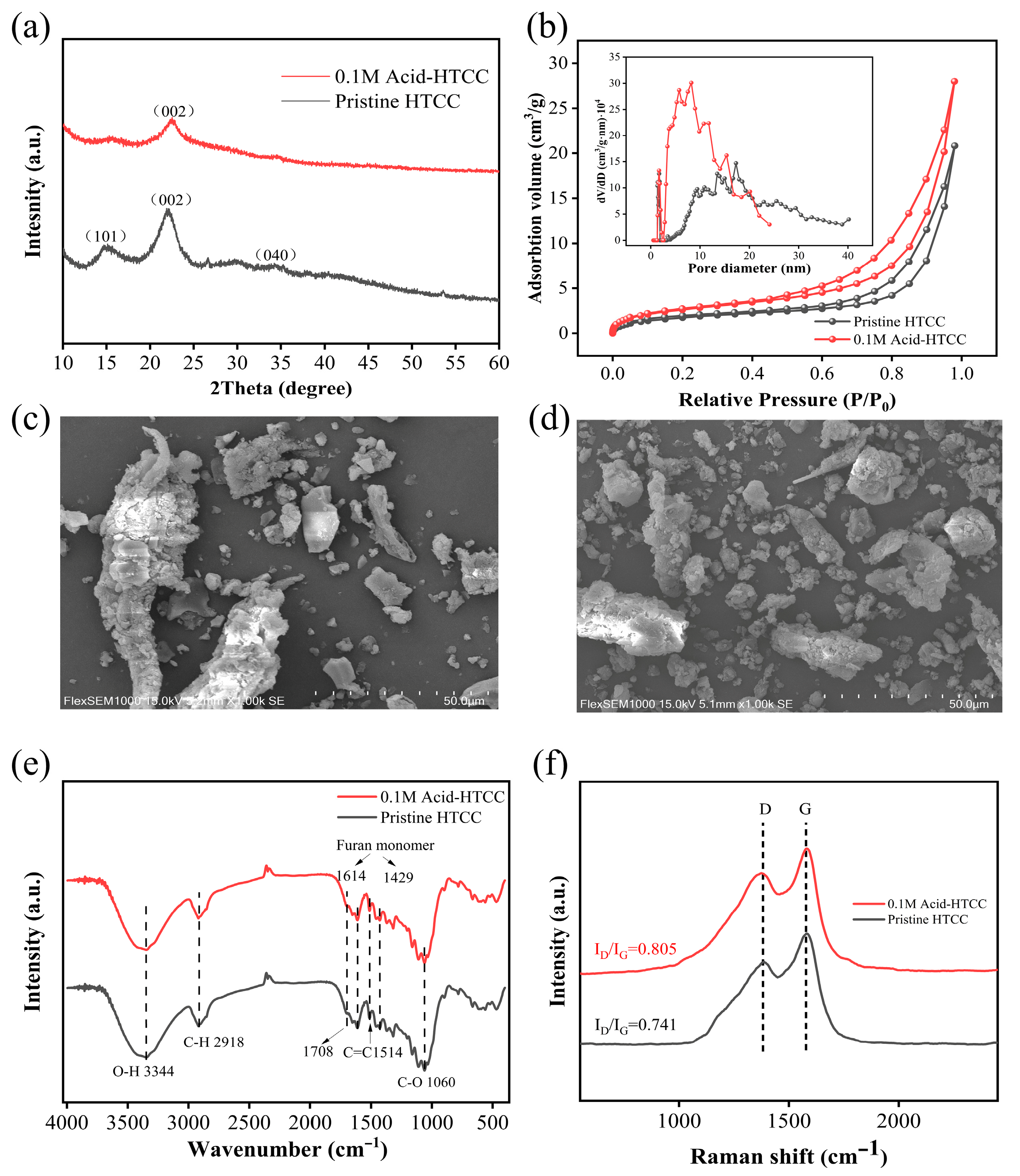
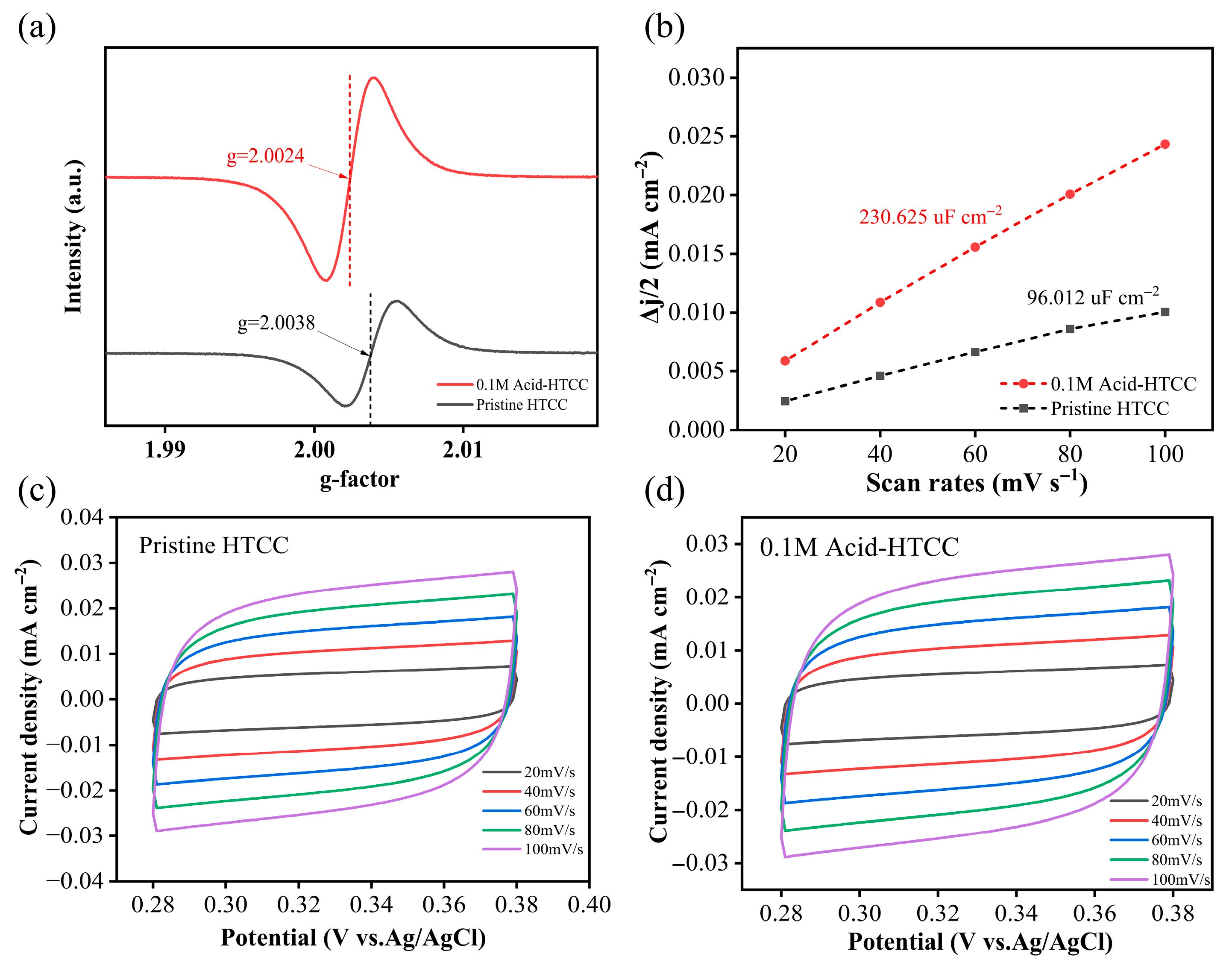
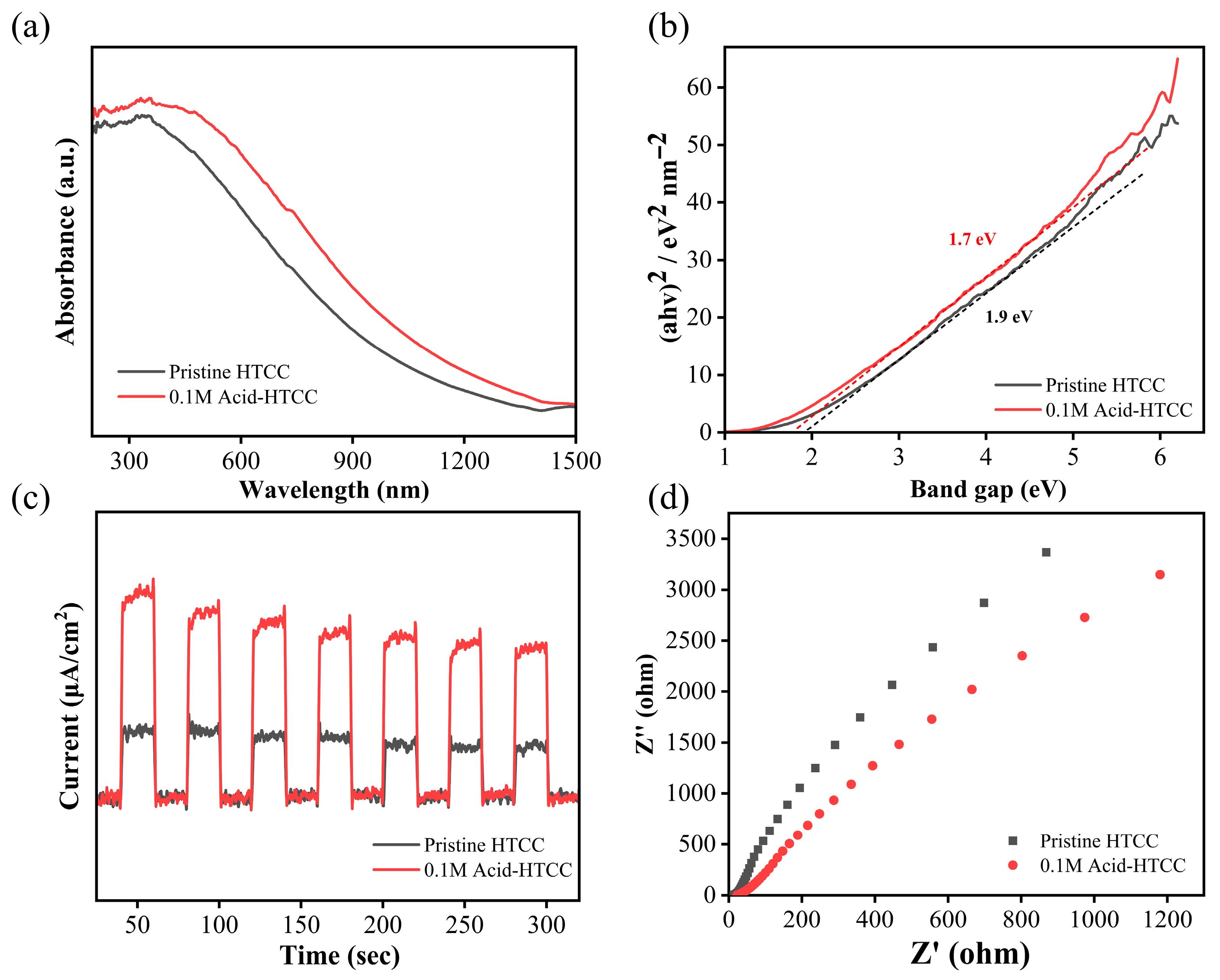
2.1. Structure and Morphology
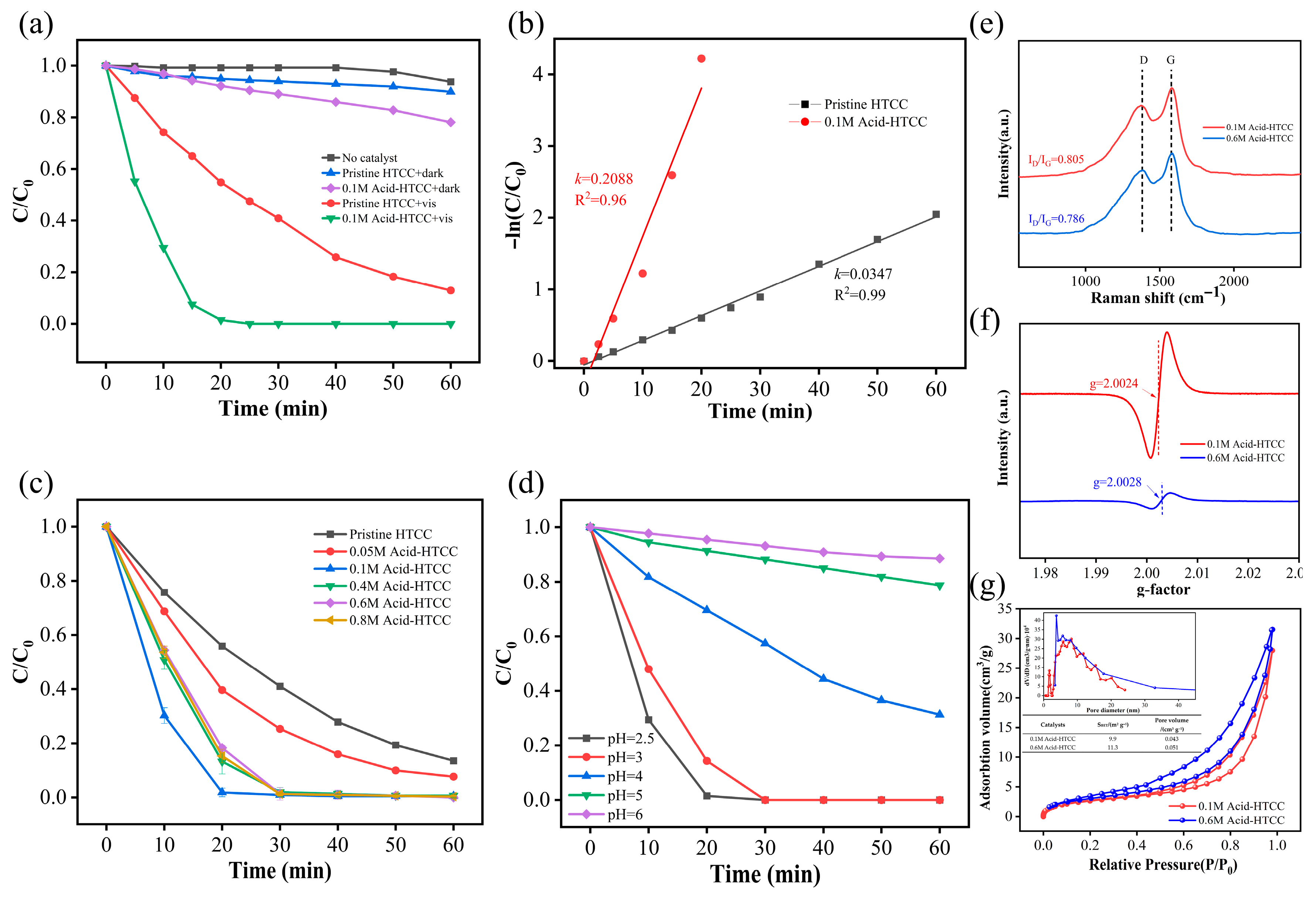
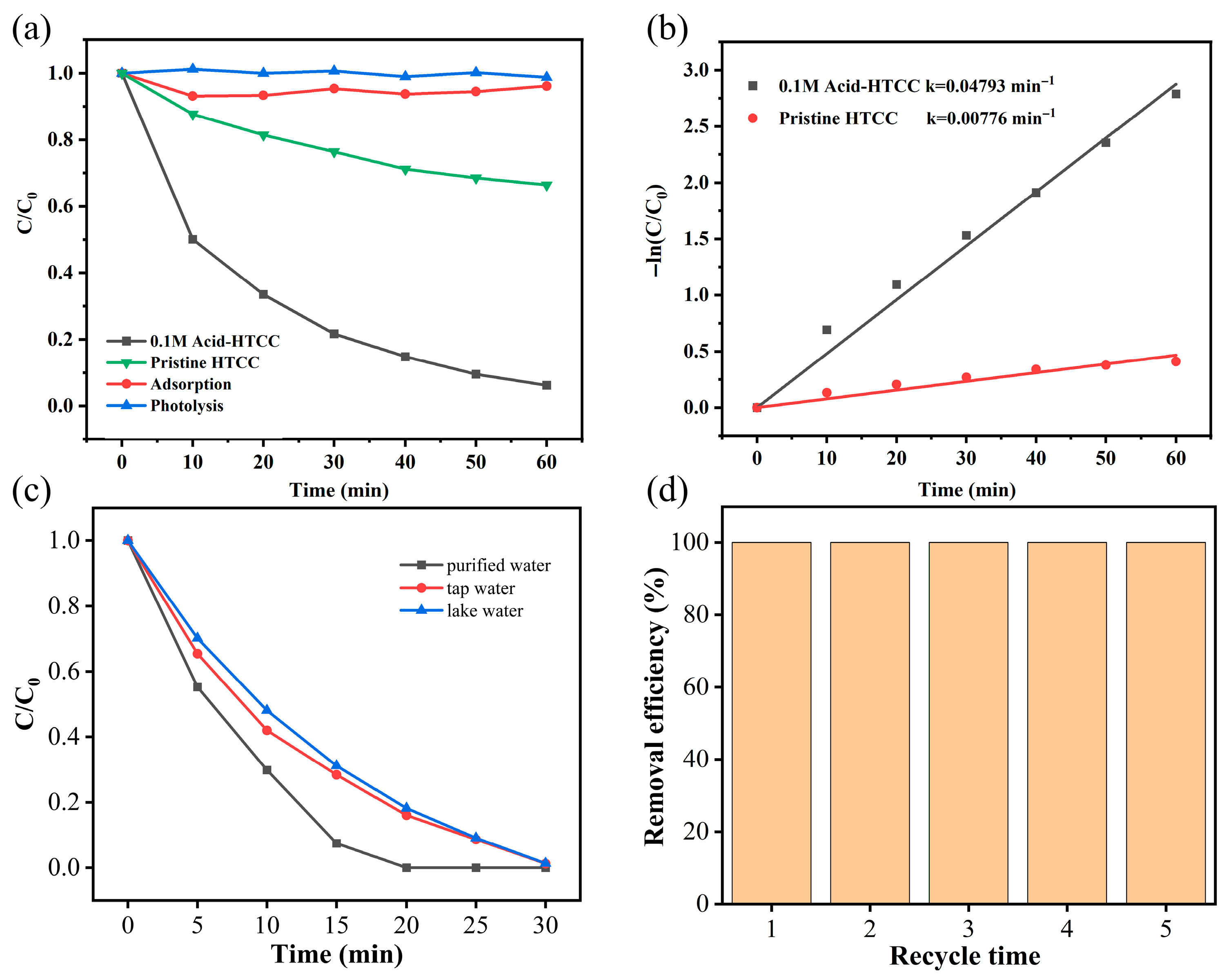
2.2. Photocatalytic Reduction of Cr(VI)
2.3. The Influence of pH
2.4. Photocatalytic Degradation of Microcystin-LR
2.5. The Influence of Water Matrix
2.6. Catalyst Stability
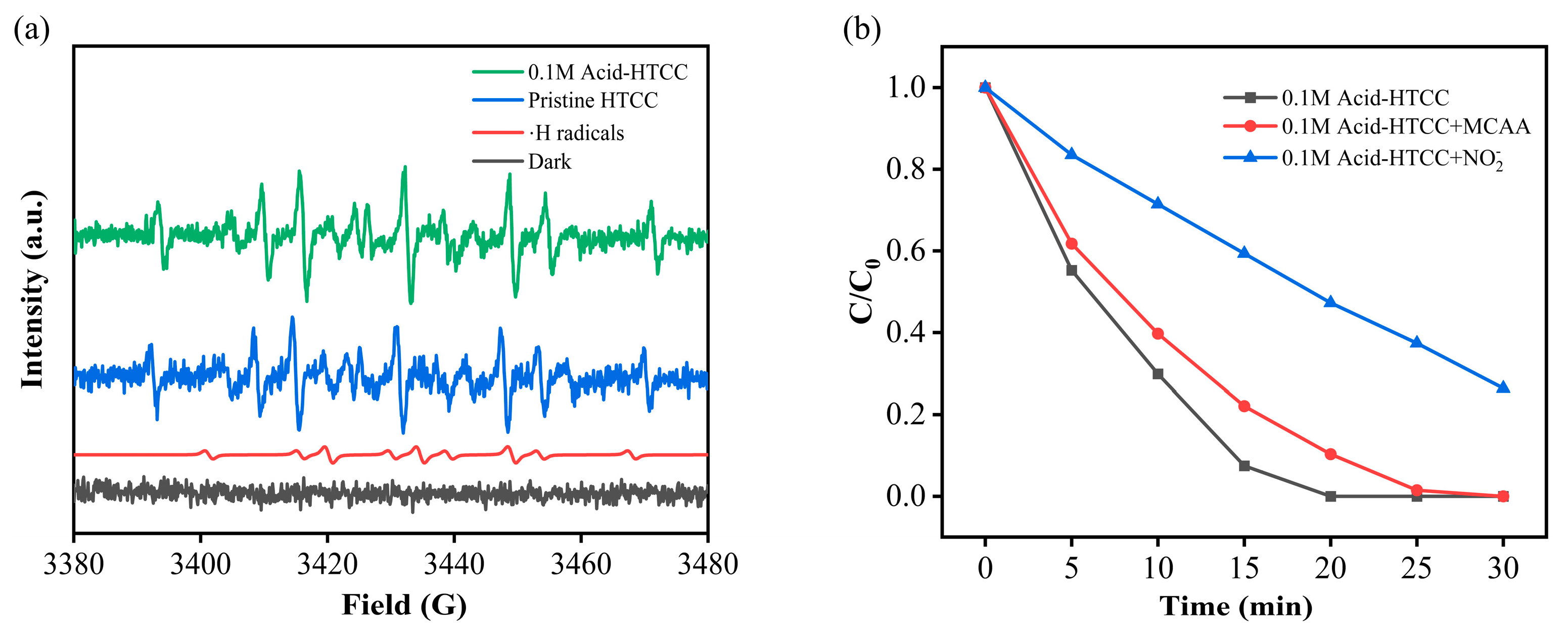
2.7. Photocatalytic Mechanism in Cr(VI) Reduction
3. Materials and Methods
3.1. Chemical Reagents
3.2. Synthesis of Catalysts
3.3. Characterization
3.4. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Gu, Y.; Fang, Y.; Johnson, D.; Chen, C.; Chen, J.; Tian, H.; Huang, Y. Self-assembled BiVO4 mesocrystals for efficient photocatalytic decontamination of microcystin-LR. Environ. Chem. Lett. 2022, 20, 1595–1601. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, F.; Tang, B. Adsorption and redox conversion behaviors of Cr(VI) on goethite/carbon microspheres and akageneite/carbon microspheres composites. Chem. Eng. J. 2019, 356, 151–160. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Xue, W.; Lei, L.; Chen, S.; Zhou, C.; Liu, X.; Wen, X.; Li, B. Eco-friendly remediation for lead-contaminated riverine sediment by sodium lignin sulfonate stabilized nano-chlorapatite. Chem. Eng. J. 2020, 397, 125396. [Google Scholar] [CrossRef]
- Bashir, M.S.; Ramzan, N.; Najam, T.; Abbas, G.; Gu, X.; Arif, M.; Qasim, M.; Bashir, H.; Shah, S.S.A.; Sillanpää, M. Metallic nanoparticles for catalytic reduction of toxic hexavalent chromium from aqueous medium: A state-of-the-art review. Sci. Total Environ. 2022, 829, 154475. [Google Scholar] [CrossRef]
- Jangi, S.H.; Khoobi, A. Detection of cadmium heavy metal ions using a nanostructured green sensor in food, biological and environmental samples. Food Chem. 2024, 458, 140307. [Google Scholar] [CrossRef]
- Singh, P.; Itankar, N.; Patil, Y. Biomanagement of hexavalent chromium: Current trends and promising perspectives. J. Environ. Manag. 2021, 279, 111547. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Xu, X.; Sui, Y.; Chen, W.; Li, X.; Huang, W.; Chai, L.; Li, Y.; Zhong, H. Photocatalytic Cr(VI) reduction by metal-free photocatalysts under visible-light irradiation. J. Environ. Chem. Eng. 2024, 12, 114306. [Google Scholar] [CrossRef]
- Ren, X.; Mao, M.; Feng, M.; Peng, T.; Long, X.; Yang, F. Fate, abundance and ecological risks of microcystins in aquatic environment: The implication of microplastics. Water Res. 2024, 251, 121121. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Hu, C.; Dittmann, E.; Song, L.; Gan, N. The biological functions of microcystins. Water Res. 2024, 262, 122119. [Google Scholar] [CrossRef] [PubMed]
- Woolway, R.L.; Sharma, S.; Smol, J.P. Lakes in hot water: The impacts of a changing climate on aquatic ecosystems. Bioscience 2022, 72, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, C.; Luo, X.; Zhang, H.; Zhang, J.; Zheng, Z. Combined toxic effects of anatoxin-a and microcystin-LR on submerged macrophytes and biofilms. J. Hazard. Mater. 2020, 389, 122053. [Google Scholar] [CrossRef]
- Nawaz, M.; Moztahida, M.; Kim, J.; Shahzad, A.; Jang, J.; Miran, W.; Lee, D.S. Photodegradation of microcystin-LR using graphene-TiO2/sodium alginate aerogels. Carbohydr. Polym. 2018, 199, 109–118. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, S.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; Pastrana-Martínez, L.M.; Likodimos, V.; Falaras, P.; Silva, A.M.T.; Hiskia, A. Photocatalytic degradation of microcystin-LR and off-odor compounds in water under UV-A and solar light with a nanostructured photocatalyst based on reduced graphene oxide-TiO2 composite. Identification of intermediate products. Ind. Eng. Chem. Res. 2013, 52, 13991–14000. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, H.; Zhao, H.; Wang, Q. Microcystin biosynthesis and toxic effects. Algal Res. 2021, 55, 102277. [Google Scholar] [CrossRef]
- Yue, L.; Tao, M.; Xu, L.; Wang, C.; Xu, Y.; Liu, Y.; Cao, X.; White, J.C.; Wang, Z. Size-dependent photocatalytic inactivation of microcystis aeruginosa and degradation of microcystin by a copper metal organic framework. J. Hazard. Mater. 2024, 462, 132799. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Yuan, C.; Huang, H.; Xiong, X.; Wu, H.; Wang, D. Efficient simultaneous removal of phosphate and microcystin-LR in water using a novel g-C3N4/calcite composite under dark-visible light cycles. Chem. Eng. J. 2024, 499, 156490. [Google Scholar] [CrossRef]
- Zhan, M.-M.; Hong, Y. Recent advances in technologies for removal of microcystins in water: A review. Curr. Pollut. Rep. 2022, 8, 113–127. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Chen, Y.; Zhuang, Z.; Chen, F.-F.; Zhu, Y.-J.; Yu, Y. Recycling heavy metals from wastewater for photocatalytic CO2 reduction. Chem. Eng. J. 2020, 402, 125922. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Li, Y.; Bian, Y.; Qin, H.; Zhang, Y.; Bian, Z. Photocatalytic reduction behavior of hexavalent chromium on hydroxyl modified titanium dioxide. Appl. Catal. B-Environ. 2017, 206, 293–299. [Google Scholar] [CrossRef]
- Choi, Y.; Mehrotra, R.; Lee, S.-H.; Nguyen, T.V.T.; Lee, I.; Kim, J.; Yang, H.-Y.; Oh, H.; Kim, H.; Lee, J.-W.; et al. Bias-free solar hydrogen production at 19.8 mA cm−2 using perovskite photocathode and lignocellulosic biomass. Nat. Commun. 2022, 13, 5709. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Wang, G.; Bi, W.; Zhang, Q.; Dong, X.; Zhang, X. Hydrothermal carbonation carbon-based photocatalysis under visible light: Modification for enhanced removal of organic pollutant and novel insight into the photocatalytic mechanism. J. Hazard. Mater. 2022, 426, 127821. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, Z.; Ma, L.; Tao, R. Biomass-derived hydrothermal carbonization carbon: A carbon-negative photocatalytic platform. Bioresour. Technol. 2025, 440, 133405. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Ou, Y.; Li, S.; Zheng, X.; Li, X.; Tang, C.; Chen, D. The reconstitution of reed cellulose by the hydrothermal carbonization and acid etching to improve the performance of photocatalytic degradation of antibiotics. Int. J. Biol. Macromol. 2023, 236, 123976. [Google Scholar] [CrossRef] [PubMed]
- Mainali, K.; Mood, S.H.; Pelaez-Samaniego, M.R.; Sierra-Jimenez, V.; Garcia-Perez, M. Production and applications of N-doped carbons from bioresources: A review. Catal. Today 2023, 423, 114248. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Dong, X.; Zhang, X. Effect of accompanying anion on the formation pathway and photocatalytic performance of metal-modified hydrothermal carbon. Sep. Purif. Technol. 2024, 341, 126872. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, Y.; Han, C.; Gui, H.; Yao, C.; Ni, C.; Li, X. Integrating biomass and minerals into photocatalysts for efficient photocatalytic N2 fixation coupled with biomass conversion. Green Chem. 2023, 25, 8706–8717. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Li, X.; Tang, C.; Li, M.; Li, S. Persulfate assisted photocatalytic degradation activity and mechanism of tetracycline by Cu enriched reed hydrothermal carbonization carbon: Synergistic effect and DFT calculation. Ind. Crop. Prod. 2023, 206, 117645. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, Z.; Yu, J.C. Converting carbohydrates to carbon-based photocatalysts for environmental treatment. Environ. Sci. Technol. 2017, 51, 7076–7083. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Z.; An, T.; Li, G.; Zhao, H.; Wong, P.K. Enhanced visible-light-driven photocatalytic bacterial inactivation by ultrathin carbon-coated magnetic cobalt ferrite nanoparticles. Environ. Sci. Technol. 2018, 52, 4774–4784. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Hu, Z.; Yu, J.C. Converting cellulose waste into a high-efficiency photocatalyst for Cr(VI) reduction via molecular oxygen activation. Appl. Catal. B-Environ. 2021, 295, 120253. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Wang, G.; Wang, X.; Zhang, X.; Dong, X. Hydrothermal carbonization carbon derived from crop straw for efficient photocatalytic elimination of antibiotics: Promotion role of native trace metals on photocatalytic performance. J. Environ. Chem. Eng. 2024, 12, 111959. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure. Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sardar, P.S.; Ghosh, S.; Biswas, M.; Ballav, N. Highly conductive polyfuran-13X zeolite-polyaniline composite. Polym. J. 2008, 40, 1199–1203. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, W. Conversion of biomasses and copper into catalysts for photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2020, 12, 51366–51373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Li, Y.; Shuai, D. Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Appl. Catal. B-Environ. 2019, 248, 11–21. [Google Scholar] [CrossRef]
- Wen, G.; Diao, J.; Wu, S.; Yang, W.; Schlögl, R.; Su, D.S. Acid properties of nanocarbons and their application in oxidative dehydrogenation. ACS Catal. 2015, 5, 3600–3608. [Google Scholar] [CrossRef]
- Bacsa, W.S.; Lannin, J.S.; Pappas, D.L.; Cuomo, J.J. Raman scattering of laser-deposited amorphous carbon. Phys. Rev. B 1993, 47, 10931. [Google Scholar] [CrossRef]
- Jäger, C.; Henning, T.; Schlögl, R.; Spillecke, O. Spectral properties of carbon black. J. Non-Cryst. Solids 1999, 258, 161–179. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Chen, T.; Qiu, Y.; Xu, Z.; Yin, D. Efficient removal of several estrogens in water by Fe-hydrochar composite and related interactive effect mechanism of H2O2 and iron with persistent free radicals from hydrochar of pinewood. Sci. Total Environ. 2019, 658, 1013–1022. [Google Scholar] [CrossRef]
- Dellinger, B.; Lomnicki, S.; Khachatryan, L.; Maskos, Z.; Hall, R.W.; Adounkpe, J.; McFerrin, C.; Truong, H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. 2007, 31, 521–528. [Google Scholar] [CrossRef]
- Lian, X.; Chen, S.; He, F.; Dong, S.; Liu, E.; Li, H.; Xu, K. Photocatalytic degradation of ammonium dinitramide over novel S-scheme g-C3N4/BiOBr heterostructure nanosheets. Sep. Purif. Technol. 2022, 286, 120449. [Google Scholar] [CrossRef]
- Zhang, J.; Balasubramanian, R.; Yang, X. Novel 3D multi-layered carbon nitride/indium sulfide heterostructure for boosted superoxide anion radical generation and enhanced photocatalysis under visible light. Chem. Eng. J. 2023, 453, 139776. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Liu, G.; Shen, Z.; Xia, D.; Wong, P.K.; Yu, J.C. Monoclinic dibismuth tetraoxide: A new visible-light-driven photocatalyst for environmental remediation. Appl. Catal. B-Environ. 2015, 176–177, 444–453. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Guo, T.; Li, J.; Wu, X.; Zhang, G. Construction of 2D/2D Bi2Se3/g-C3N4 nanocomposite with high interfacial charge separation and photo-heat conversion efficiency for selective photocatalytic CO2 reduction. Appl. Catal. B-Environ. 2020, 277, 119232. [Google Scholar] [CrossRef]
- He, X.; Wang, A.; Wu, P.; Tang, S.; Zhang, Y.; Li, L.; Ding, P. Photocatalytic degradation of microcystin-LR by modified TiO2 photocatalysis: A review. Sci. Total Environ. 2020, 743, 140694. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Tang, C.; Wen, S.; Wu, X.; Long, J.; Yang, X.; Wang, H.; Zhou, L. Mechanisms underlying degradation pathways of microcystin-LR with doped TiO2 photocatalysis. Chem. Eng. J. 2017, 330, 355–371. [Google Scholar] [CrossRef]
- Zetterholm, S.G.; Gurtowski, L.; Roberts, J.L.; McLeod, S.; Fernando, B.M.; Griggs, C.S. Graphene-mediated removal of microcystin-LR in chitosan/graphene composites for treatment of harmful algal blooms. Chemosphere 2022, 300, 134583. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xiao, D.; Ji, L.; Jin, D.; Dai, K.; Yang, Z.; Wang, S.; Chen, H. Simple fabrication of Fe3O4/C/g-C3N4 two-dimensional composite by hydrothermal carbonization approach with enhanced photocatalytic performance under visible light. Catal. Sci. Technol. 2018, 14, 3484–3492. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Tian, C.; Zhang, H.; Li, X.; Liu, W.; Li, W.; Kuang, S.; Tong, H. Boosted photocatalytic removal of Cr(VI) using MoS2 modified g-C3N4/ZnFe2O4 magnetic heterojunction composites. Process Saf. Environ. Protect. 2022, 162, 72–82. [Google Scholar] [CrossRef]
- Hu, J.; Lu, S.; Ma, J.; Zhu, F.; Komarneni, S. Composite of g-C3N4/ZnIn2S4 for efficient adsorption and visible light photocatalytic reduction of Cr(VI). Environ. Sci. Pollut. Res. 2022, 29, 76404–76416. [Google Scholar] [CrossRef]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B-Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, H.; Jiang, S.; Wang, C.; Xu, X.; Wang, L. Construction of precious metal-loaded BiOI semiconductor materials with improved photocatalytic activity for microcystin-LR degradation. Environ. Sci. Pollut. Res. 2019, 26, 8226–8236. [Google Scholar] [CrossRef]
- Peng, J.; Du, K.; Sun, J.; Yang, X.; Wang, X.; Zhang, X.; Song, G.; Feng, F. Photocatalytic generation of hydrogen radical (H·) with GSH for photodynamic therapy. Angew. Chem. Int. Ed. 2023, 62, e202214991. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef]
| Catalysts | SBET/(m2 g−1) | Pore Volume/(cm3 g−1) | Pore Size/(nm) |
|---|---|---|---|
| Pristine HTCC | 6.6 | 0.032 | 17.3 |
| 0.1 M Acid-HTCC | 9.9 | 0.043 | 8.3 |
| Scavenger | Rate Constants (mol−1 s−1) | |
|---|---|---|
| eaq− | ·H | |
| NO2- | 3.5 × 109 | 7.1 × 108 |
| MCAA | 6.9 × 109 | 6.5 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Chen, X.; Wan, Z.; Chen, Y. Synthesis of Straw-Based Hydrothermal Carbonation Carbon and Its Photocatalytic Removal of Cr(VI) and Microcystin-LR. Molecules 2025, 30, 4399. https://doi.org/10.3390/molecules30224399
Luo Y, Chen X, Wan Z, Chen Y. Synthesis of Straw-Based Hydrothermal Carbonation Carbon and Its Photocatalytic Removal of Cr(VI) and Microcystin-LR. Molecules. 2025; 30(22):4399. https://doi.org/10.3390/molecules30224399
Chicago/Turabian StyleLuo, Yu, Xunxian Chen, Zhen Wan, and Yingming Chen. 2025. "Synthesis of Straw-Based Hydrothermal Carbonation Carbon and Its Photocatalytic Removal of Cr(VI) and Microcystin-LR" Molecules 30, no. 22: 4399. https://doi.org/10.3390/molecules30224399
APA StyleLuo, Y., Chen, X., Wan, Z., & Chen, Y. (2025). Synthesis of Straw-Based Hydrothermal Carbonation Carbon and Its Photocatalytic Removal of Cr(VI) and Microcystin-LR. Molecules, 30(22), 4399. https://doi.org/10.3390/molecules30224399





