Decomposition Dynamics of a New Noble-Gas Compound
Abstract
1. Introduction
2. Results and Discussion
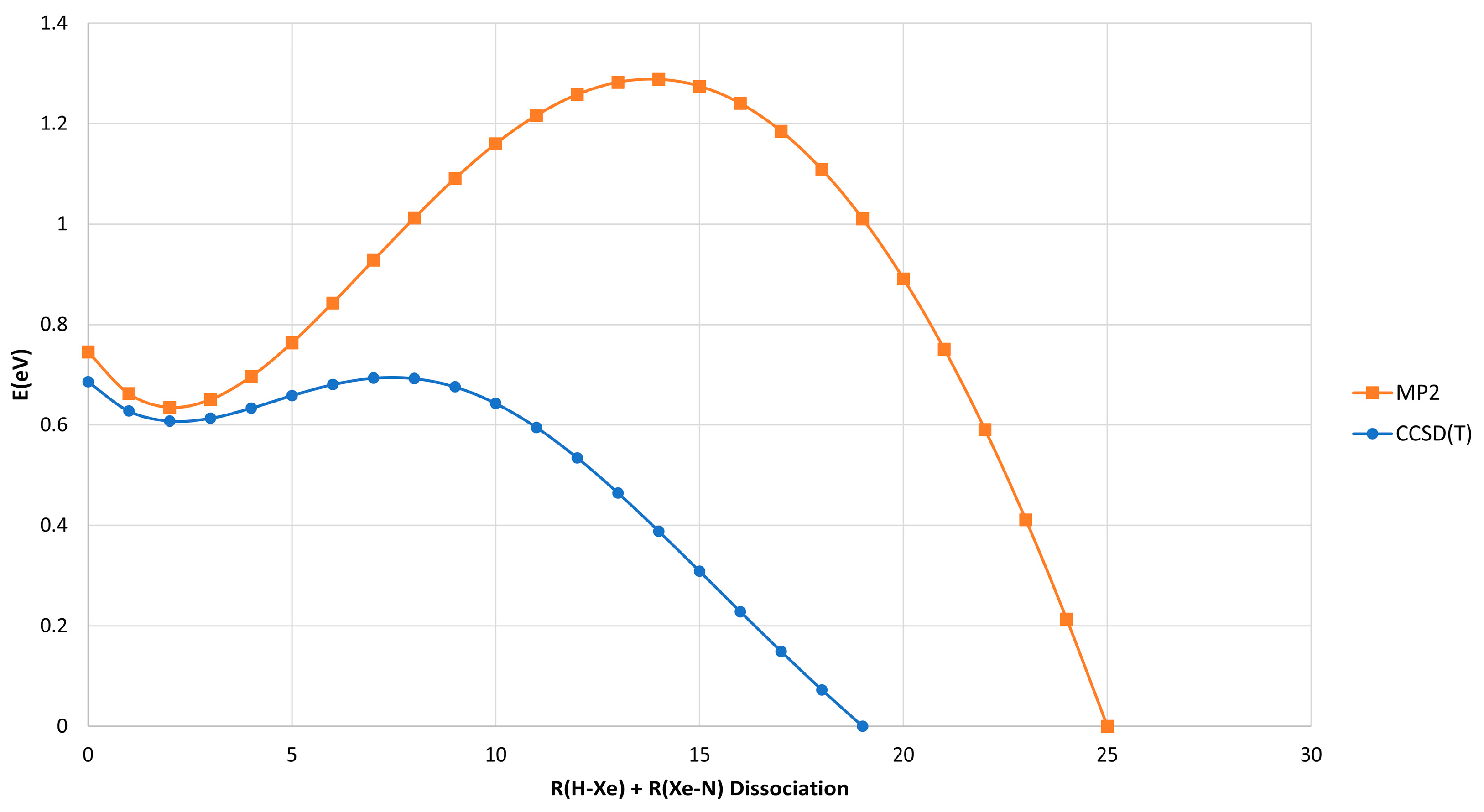
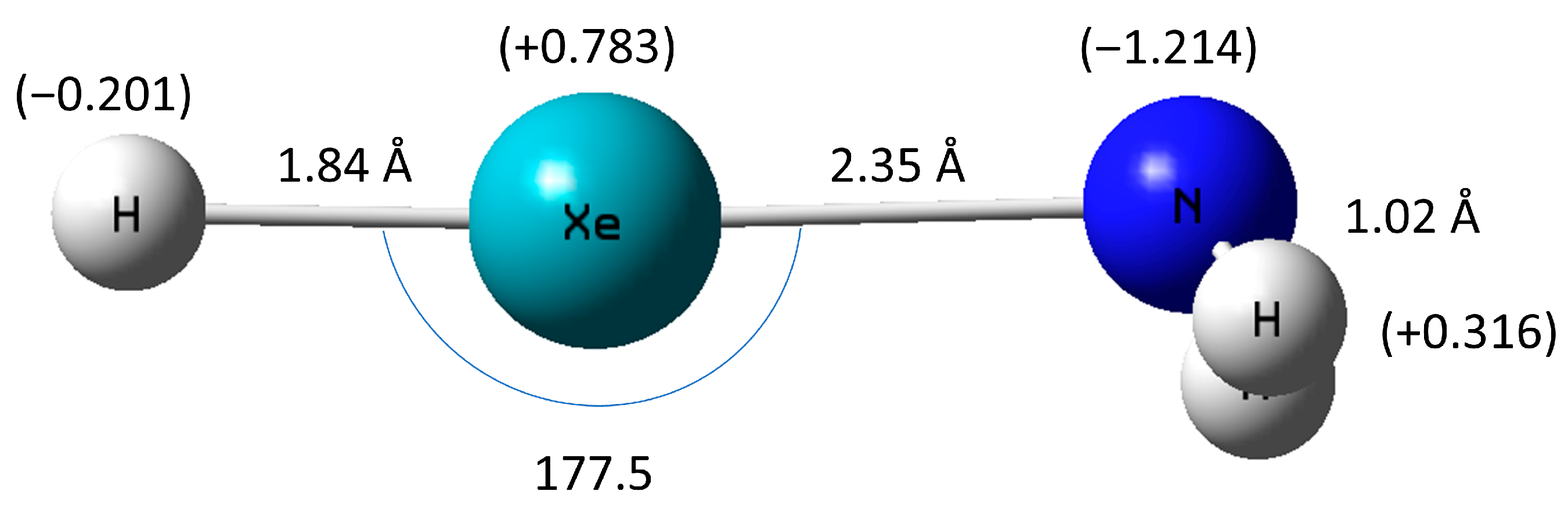
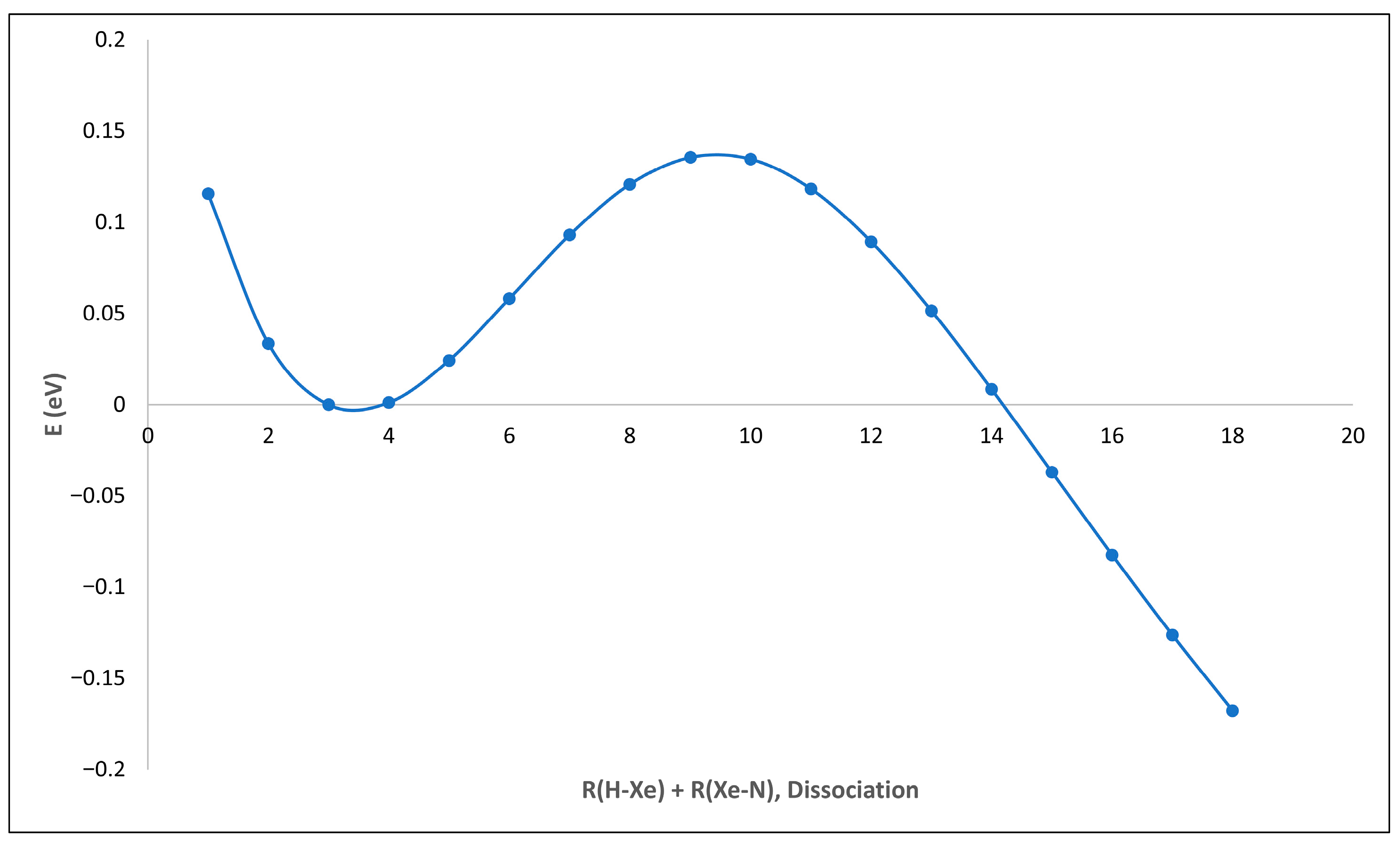
2.1. HXeNH2: Structure and Properties
2.2. HXeNH2: Decomposition Dynamics at Cryogenic Temperatures
3. Computational Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ng | Noble gas |
| AIMD | Ab Initio Molecular Dynamics |
| BOMD | Born–Oppenheimer Molecular Dynamics |
| DOF | Degrees of Freedom |
| NBO | Natural Bond Orbital |
| ECP | Effective Core Potential |
| CCSD(T) | Coupled Cluster of Singles Doubles and perturbative Triples |
| MRCI | Multi-reference Configuration Interaction |
| ZPE | Zero-Point Energy |
| LJ-18 | LaJohn-18 ECP basis-set |
| MP2 | Møller–Plesset 2nd order perturbation theory |
Appendix A
| Mode | CCSD(T) Freq. (cm−1) | Mode Description |
|---|---|---|
| 1 | 324.04 | Xe-NH2 Str. |
| 2 | 515.76 | Bend |
| 3 | 537.80 | Bend |
| 4 | 785.82 | Bend |
| 5 | 821.70 | H + NH2 Symm. bend |
| 6 | 1318.50 | H-Xe Str. + NH2 Symm. bend |
| 7 | 1580.88 | H-Xe str. + NH2 Symm. bend |
| 8 | 3421.97 | NH2 Symm. Str. |
| 9 | 3509.04 | NH2 Asymm. Str. |
| T | Number of Trajectories | Duration | General Analysis Every nth Snapshot | Number of Calculated NBO Snapshots (Every Number fs) |
|---|---|---|---|---|
| 8 K | 2 | ~18 ps | 4 | 144 (125 fs) |
| 1 | ~150 ps | 10 | 250 (175 fs) | |
| 20 K | 2 | ~18 ps | 4 | |
| 1 | ~150 ps | 10 | 250 (77 fs) | |
| 40 K | 2 | ~18 ps | 4 | 144 (125 fs) |
| 1 | ~54 ps | 4 | 216 (125 fs) | |
| 60 K | 2 | ~18 ps | 4 |
| Atom | X | Y | Z |
|---|---|---|---|
| N | 0.000000 | 0.000000 | 0.000000 |
| Xe | 0.000000 | 0.000000 | 2.349089 |
| H | 0.080033 | 0.000000 | 4.191635 |
| H | −0.618583 | 0.777530 | −0.228302 |
| H | −0.556748 | −0.823225 | −0.227285 |

References
- Grochala, W. Atypical Compounds of Gases, Which Have Been Called ‘Noble’. Chem. Soc. Rev. 2007, 36, 1632–1655. [Google Scholar] [CrossRef]
- Pettersson, M.; Lundell, J.; Räsänen, M. Neutral Rare-gas Containing Charge-transfer Molecules in Solid Matrices. I. HXeCl, HXeBr, HXeI, and HKrCl in Kr and Xe. J. Chem. Phys. 1995, 102, 6423–6431. [Google Scholar] [CrossRef]
- Grochala, W.; Khriachtchev, L.; Räsänen, M. Noble-Gas Chemistry. In Physics & Chemistry at Low Temperatures; Khriachtchev, L., Ed.; Pan Stanford Publishing: Singapore, 2011; pp. 421–448. [Google Scholar]
- Lignell, A.; Lundell, J.; Khriachtchev, L.; Räsänen, M. Experimental and Computational Study of HXeY⋯HX Complexes (X, Y = Cl and Br): An Example of Exceptionally Large Complexation Effect. J. Phys. Chem. A 2008, 112, 5486–5494. [Google Scholar] [CrossRef]
- Lignell, A.; Khriachtchev, L. Intermolecular Interactions Involving Noble-Gas Hydrides: Where the Blue Shift of Vibrational Frequency Is a Normal Effect. J. Mol. Struct. 2008, 889, 1–11. [Google Scholar] [CrossRef]
- Lignell, A.; Khriachtchev, L.; Pettersson, M.; Räsänen, M. Interaction of Rare-Gas-Containing Molecules with Nitrogen: Matrix-Isolation and Ab Initio Study of HArF…N2, HKrF⋯N2, and HKrCl⋯N2 Complexes. J. Chem. Phys. 2003, 118, 11120–11128. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Pettersson, M.; Runeberg, N.; Lundell, J.; Räsänen, M. A Stable Argon Compound. Nature 2000, 406, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Yockel, S.; Seals, J.J.; Wilson, A.K. An Ab Initio Study of the Noble Gas Compound HKrCl. Chem. Phys. Lett. 2004, 393, 448–452. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Tanskanen, H.; Lundell, J.; Pettersson, M.; Kiljunen, H.; Räsänen, M. Fluorine-Free Organoxenon Chemistry: HXeCCH, HXeCC, and HXeCCXeH. J. Am. Chem. Soc. 2003, 125, 4696–4697. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Tanskanen, H.; Cohen, A.; Gerber, R.B.; Lundell, J.; Pettersson, M.; Kiljunen, H.; Räsänen, M. A Gate to Organokrypton Chemistry: HKrCCH. J. Am. Chem. Soc. 2003, 125, 6876–6877. [Google Scholar] [CrossRef]
- Pettersson, M.; Khriachtchev, L.; Lundell, J.; Räsänen, M. A Chemical Compound Formed from Water and Xenon: HXeOH. J. Am. Chem. Soc. 1999, 121, 11904–11905. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Tanskanen, H.; Pettersson, M.; Räsänen, M.; Ahokas, J.; Kunttu, H.; Feldman, V. On Photochemistry of Water in Solid Xe: Thermal and Light-Induced Decomposition of HXeOH and HXeH and Formation of H2O2. J. Chem. Phys. 2002, 116, 5649–5656. [Google Scholar] [CrossRef]
- Feldman, V.I.; Sukhov, F.F.; Orlov, A.Y.; Tyulpina, I.V. Experimental Evidence for the Formation of HXeCCH: The First Hydrocarbon with an Inserted Rare-Gas Atom. J. Am. Chem. Soc. 2003, 125, 4698–4699. [Google Scholar] [CrossRef]
- Ryazantsev, S.V.; Kobzarenko, A.V.; Feldman, V.I. Photolabile Xenon Hydrides: A Case Study of HXeSH and HXeH. J. Chem. Phys. 2013, 139, 124315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, K.; Sheng, L. Predicted Organic Noble-Gas Hydrides Derived from Acrylic Acid. J. Phys. Chem. A 2015, 119, 2393–2400. [Google Scholar] [CrossRef]
- Lignell, A.; Khriachtchev, L.; Lundell, J.; Tanskanen, H.; Räsänen, M. On Theoretical Predictions of Noble-Gas Hydrides. J. Chem. Phys. 2006, 125, 184514. [Google Scholar] [CrossRef] [PubMed]
- Operti, L.; Rabezzana, R.; Turco, F.; Borocci, S.; Giordani, M.; Grandinetti, F. Xenon–Nitrogen Chemistry: Gas-Phase Generation and Theoretical Investigation of the Xenon–Difluoronitrenium Ion F2N-Xe+. Chem.—A Eur. J. 2011, 17, 10682–10689. [Google Scholar] [CrossRef]
- Foropoulos, J.; DesMarteau, D.D. Bis[Bis(Trifluoromethanesulfonyl)Imido]Xenon: A New Compound Possessing Xenon-Nitrogen Bonds. J. Am. Chem. Soc. 1982, 104, 4260–4261. [Google Scholar] [CrossRef]
- Sawyer, J.F.; Schrobilgen, G.J.; DesMarteau, D.D.; Foropoulos, J. Xenon-Nitrogen Bonded Compounds. J. Fluor. Chem. 1982, 21, 9. [Google Scholar] [CrossRef]
- DesMarteau, D.D.; LeBlond, R.D.; Fazley Hossain, S.; Nothe, D. Xenon-Nitrogen Bonds. The Synthesis and Characterization of [Imidobis(Sulfuryl Fluoride)]Xenon(II) Derivatives Xe[N(SO2F)2]2, FXeN(SO2F)2, and [(FSO2)2NXe]2F+AsF6− and the Radical. N(SO2F)2. J. Am. Chem. Soc. 1981, 103, 7734–7739. [Google Scholar] [CrossRef]
- Schumacher, G.A.; Schrobilgen, G.J. Synthesis, Multinuclear Magnetic Resonance, and Raman Study of 15N-Enriched Xe[N(SO2F)2]2, an Example of Xenon-Nitrogen Bonding. Solution Behavior of [15N]-F[XeN(SO2F)2]2+AsF6−. Inorg. Chem. 1983, 22, 2178–2183. [Google Scholar] [CrossRef]
- Emara, A.A.A.; Paprica, A.; Sanders, J.C.P.; Schrobilgen, G.J. Novel Bonding Situations in Noble-Gas Chemistry. J. Fluor. Chem. 1989, 45, 20. [Google Scholar] [CrossRef]
- Pettersson, M.; Khriachtchev, L.; Lundell, J.; Jolkkonen, S.; Räsänen, M. Photochemistry of HNCO in Solid Xenon: Photoinduced and Thermally Activated Formation of HXeNCO†. J. Phys. Chem. A 2000, 104, 3579–3583. [Google Scholar] [CrossRef]
- Pettersson, M.; Lundell, J.; Khriachtchev, L.; Räsänen, M. Neutral Rare-Gas Containing Charge-Transfer Molecules in Solid Matrices. III. HXeCN, HXeNC, and HKrCN in Kr and Xe. J. Chem. Phys. 1998, 109, 618–625. [Google Scholar] [CrossRef]
- Zhu, C.; Räsänen, M.; Khriachtchev, L. Fluorinated Noble-Gas Cyanides FKrCN, FXeCN, and FXeNC. J. Chem. Phys. 2015, 143, 074306. [Google Scholar] [CrossRef]
- Arppe, T.; Khriachtchev, L.; Lignell, A.; Domanskaya, A.V.; Räsänen, M. Halogenated Xenon Cyanides ClXeCN, ClXeNC, and BrXeCN. Inorg. Chem. 2012, 51, 4398–4402. [Google Scholar] [CrossRef]
- Tsivion, E.; Gerber, R.B. Stability of Noble-Gas Hydrocarbons in an Organic Liquid-like Environment: HXeCCH in Acetylene. Phys. Chem. Chem. Phys. 2011, 13, 19601–19606. [Google Scholar] [CrossRef] [PubMed]
- Tsivion, E.; Gerber, R.B. Lifetimes of Compounds Made of Noble-Gas Atoms with Water. Chem. Phys. Lett. 2009, 482, 30–33. [Google Scholar] [CrossRef]
- Cohen, A.; Gerber, R.B. Prediction of New Noble-Gas Molecules with Multiple Xe-N Bonds. SSRN 2025. [Google Scholar] [CrossRef]
- Zhao, R.; Sheng, L.; Gao, K. Theoretical Prediction of an NXeH4+ Ion with N-Xe Triple Bond. Comput. Theor. Chem. 2021, 1199, 113193. [Google Scholar] [CrossRef]
- Tolstikhin, I.N.; O’Nions, R.K. The Earth’s Missing Xenon: A Combination of Early Degassing and of Rare Gas Loss from the Atmosphere. Chem. Geol. 1994, 115, 1–6. [Google Scholar] [CrossRef]
- Callaway, E. The Mysterious Case of the Missing Noble Gas. Nature 2012. [Google Scholar] [CrossRef]
- Sanloup, C.; Schmidt, B.C.; Perez, E.M.C.; Jambon, A.; Gregoryanz, E.; Mezouar, M. Retention of Xenon in Quartz and Earth’s Missing Xenon. Science 2005, 310, 1174–1177. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, J.; Cui, W.; Liu, C.; Ding, S.; Yang, K.; Hao, J.; Li, Y. Formation of NH3-Xe Compound at the Extreme Condition of Planetary Interiors. Phys. Rev. B 2022, 105, 214109. [Google Scholar] [CrossRef]
- Sanloup, C. Noble Gas Reactivity in Planetary Interiors. Front. Phys. 2020, 8, 541891. [Google Scholar] [CrossRef]
- Howie, R.T.; Turnbull, R.; Binns, J.; Frost, M.; Dalladay-Simpson, P.; Gregoryanz, E. Formation of Xenon-Nitrogen Compounds at High Pressure. Sci. Rep. 2016, 6, 34896. [Google Scholar] [CrossRef] [PubMed]
- Sanloup, C.; Bonev, S.A.; Hochlaf, M.; Maynard-Casely, H.E. Reactivity of Xenon with Ice at Planetary Conditions. Phys. Rev. Lett. 2013, 110, 265501. [Google Scholar] [CrossRef]
- Pettersson, M.; Lundell, J.; Räsänen, M. Neutral Rare-gas Containing Charge-transfer Molecules in Solid Matrices. II. HXeH, HXeD, and DXeD in Xe. J. Chem. Phys. 1995, 103, 205–210. [Google Scholar] [CrossRef]
- Gerber, R.B. Formation of Novel Rare-Gas Molecules in Low-Temperature Matrices. Annu. Rev. Phys. Chem. 2004, 55, 55–78. [Google Scholar] [CrossRef]
- Pettersson, M.; Lundell, J.; Räsänen, M. New Rare-Gas-Containing Neutral Molecules. Eur. J. Inorg. Chem. 1999, 1999, 729–737. [Google Scholar] [CrossRef]
- Gerber, R.B.; Tsivion, E.; Khriachtchev, L.; Räsänen, M. Intrinsic Lifetimes and Kinetic Stability in Media of Noble-Gas Hydrides. Chem. Phys. Lett. 2012, 545, 1–8. [Google Scholar] [CrossRef]
- Runeberg, N.; Pettersson, M.; Khriachtchev, L.; Lundell, J.; Räsänen, M. A Theoretical Study of HArF, a Newly Observed Neutral Argon Compound. J. Chem. Phys. 2001, 114, 836–841. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Discovering Chemistry with Natural Bond Orbitals; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–319. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.02; Gaussian Inc.: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Lundell, J.; Cohen, A.; Gerber, R.B. Quantum Chemical Calculations on Novel Molecules from Xenon Insertion into Hydrocarbons. J. Phys. Chem. A 2002, 106, 11950–11955. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, version 3.1; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent Developments in the General Atomic and Molecular Electronic Structure System. J. Chem. Phys. 2020, 152, 144102. [Google Scholar] [CrossRef] [PubMed]
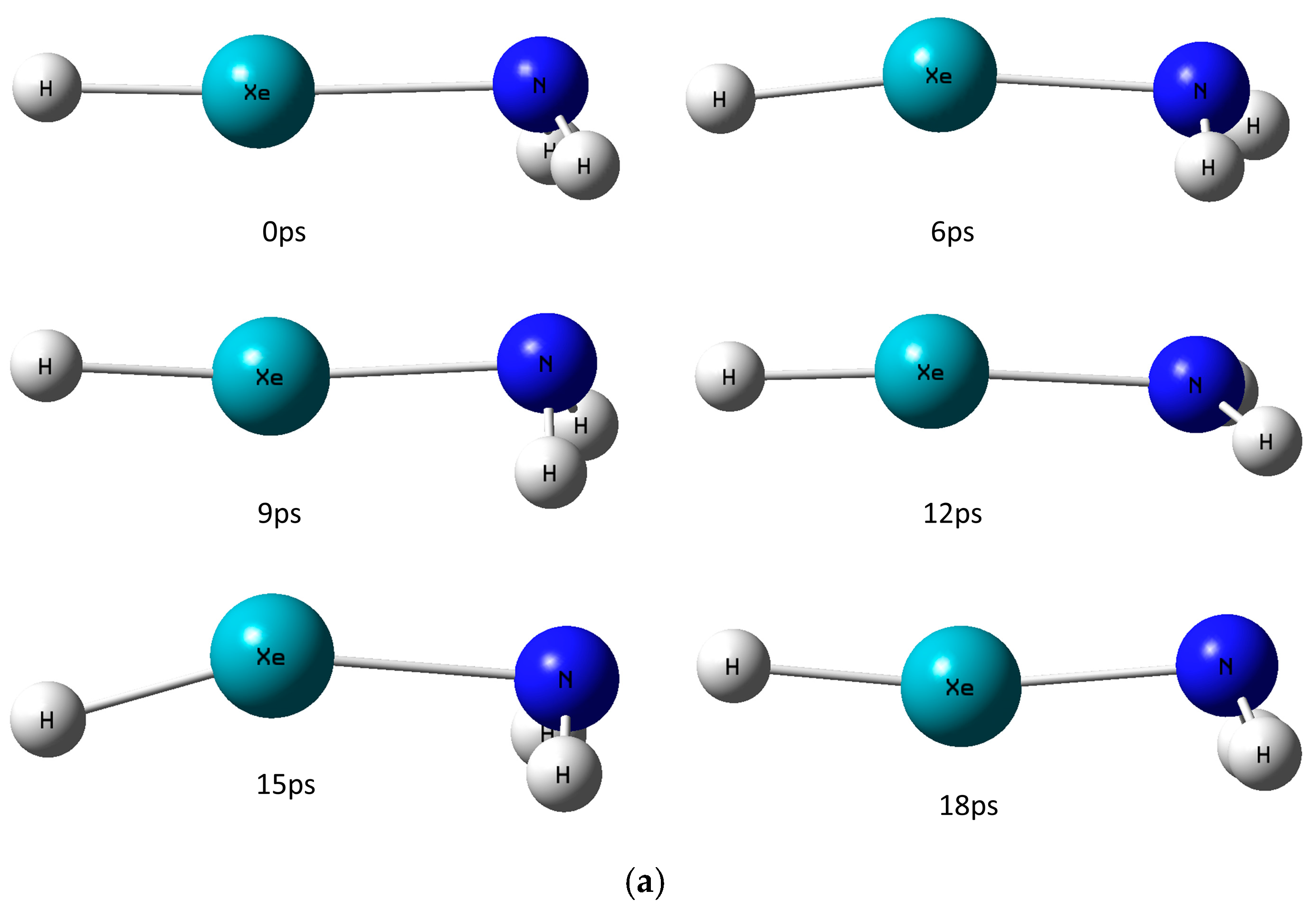
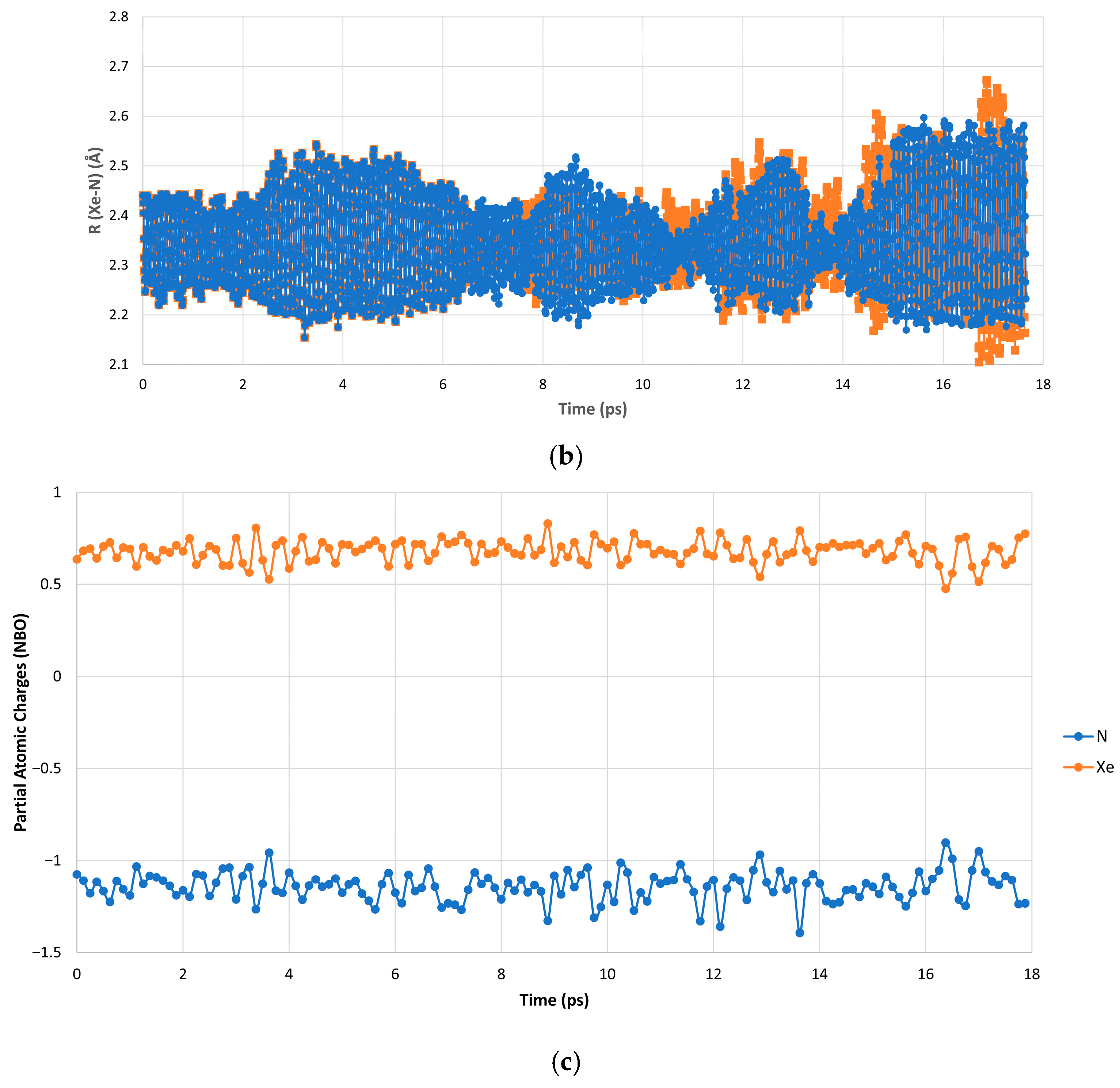
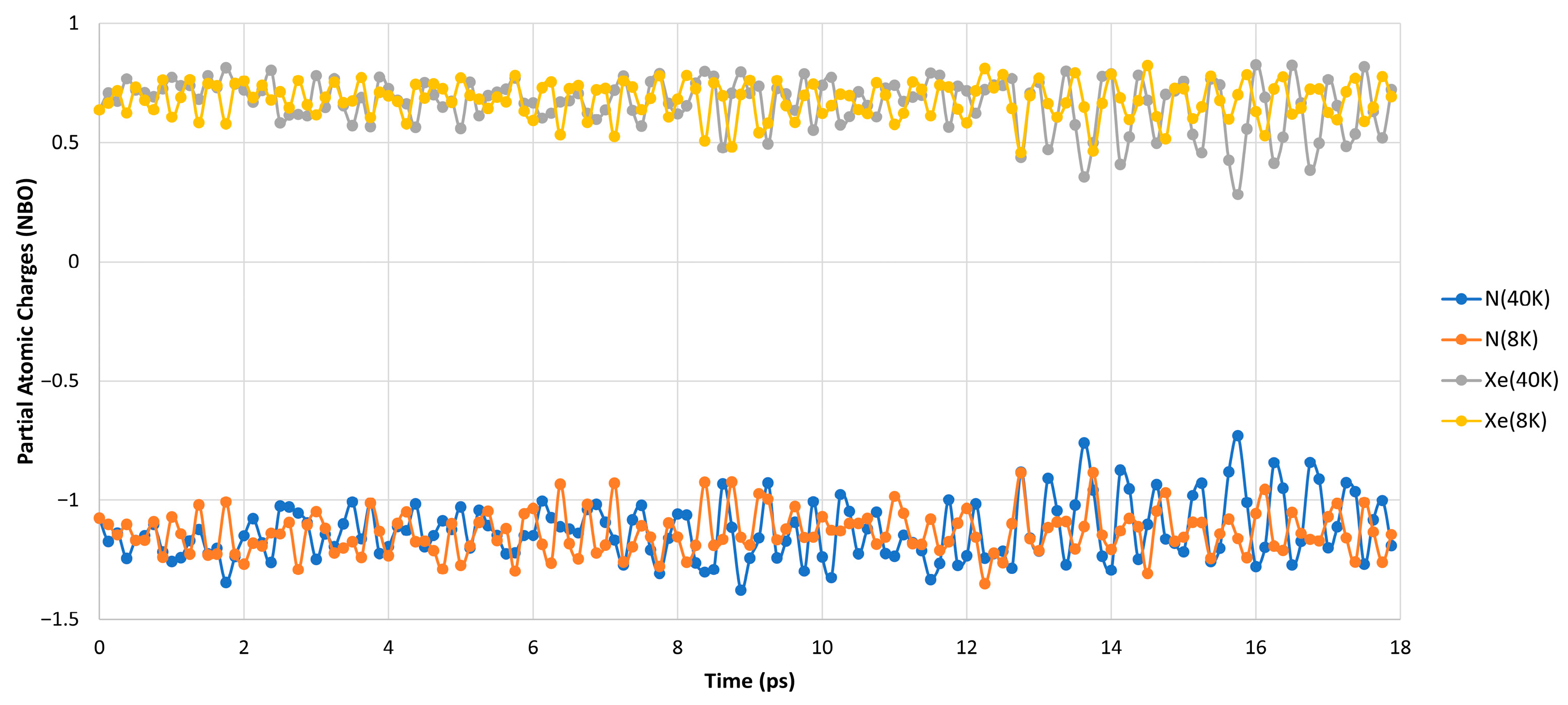
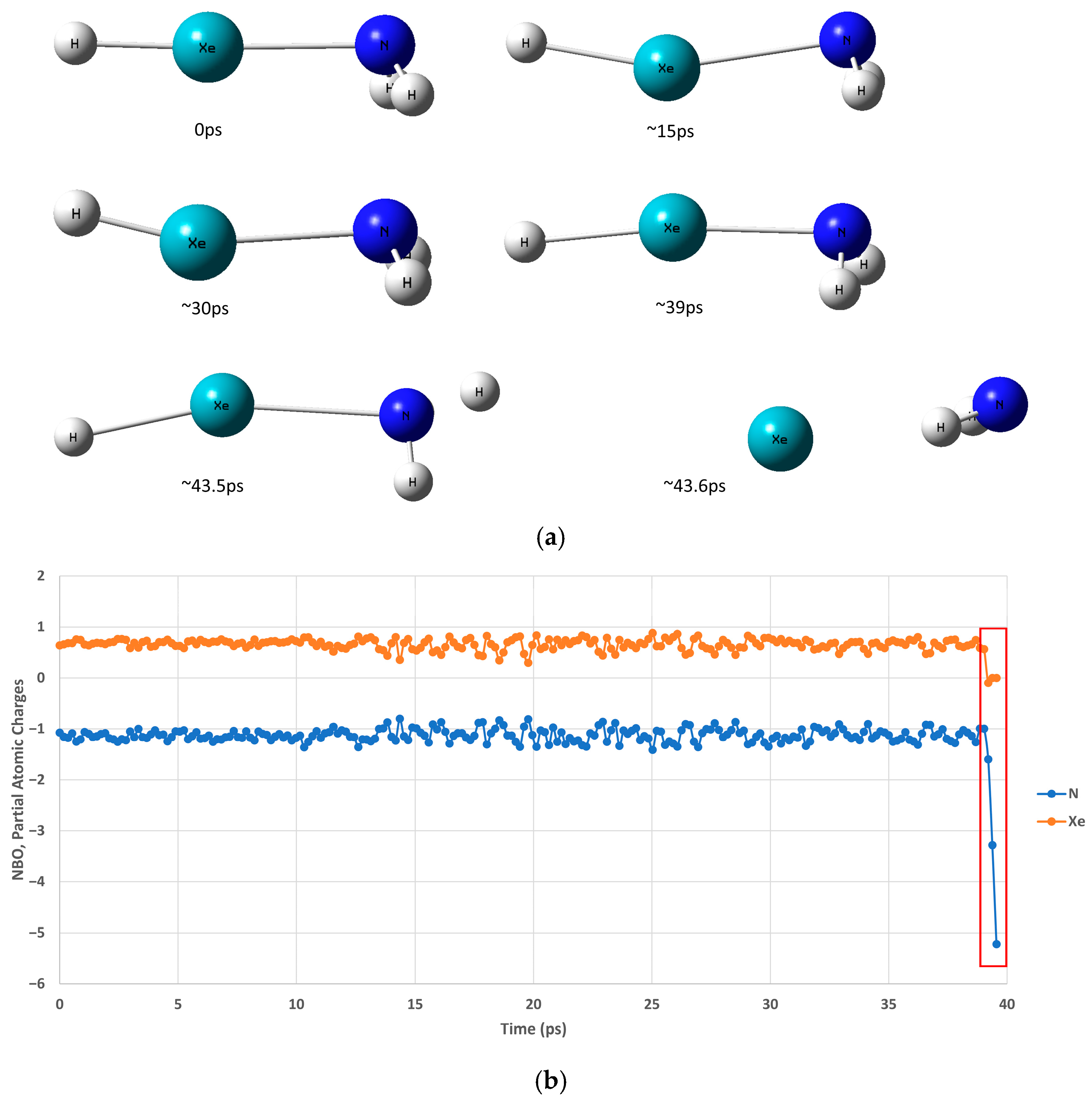
| 8 K [Min, Max, Av.] (Å/Degrees) | 20 K [Min, Max, Av.] (Å/Degrees) | |
|---|---|---|
| R (H-Xe, 1.84 Å) | [1.59, 2.15, 1.83] | [1.47, 2.21, 1.84] |
| R (Xe-N, 2.35 Å) | [2.10, 2.71, 2.36] | [2.08, 2.73, 2.36] |
| A (H-Xe-N, 177.5°) | [156.5, 179.8, 170.7] | [155.1, 179.9, 171.3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, A.; Gerber, R.B. Decomposition Dynamics of a New Noble-Gas Compound. Molecules 2025, 30, 4398. https://doi.org/10.3390/molecules30224398
Cohen A, Gerber RB. Decomposition Dynamics of a New Noble-Gas Compound. Molecules. 2025; 30(22):4398. https://doi.org/10.3390/molecules30224398
Chicago/Turabian StyleCohen, Arik, and Robert Benny Gerber. 2025. "Decomposition Dynamics of a New Noble-Gas Compound" Molecules 30, no. 22: 4398. https://doi.org/10.3390/molecules30224398
APA StyleCohen, A., & Gerber, R. B. (2025). Decomposition Dynamics of a New Noble-Gas Compound. Molecules, 30(22), 4398. https://doi.org/10.3390/molecules30224398





