Nitric Acid Leaching for Magnesium Extraction from Asbestos Ore Waste: From DoE to Predictive Modeling and Cost-Efficient Optimization
Abstract
1. Introduction
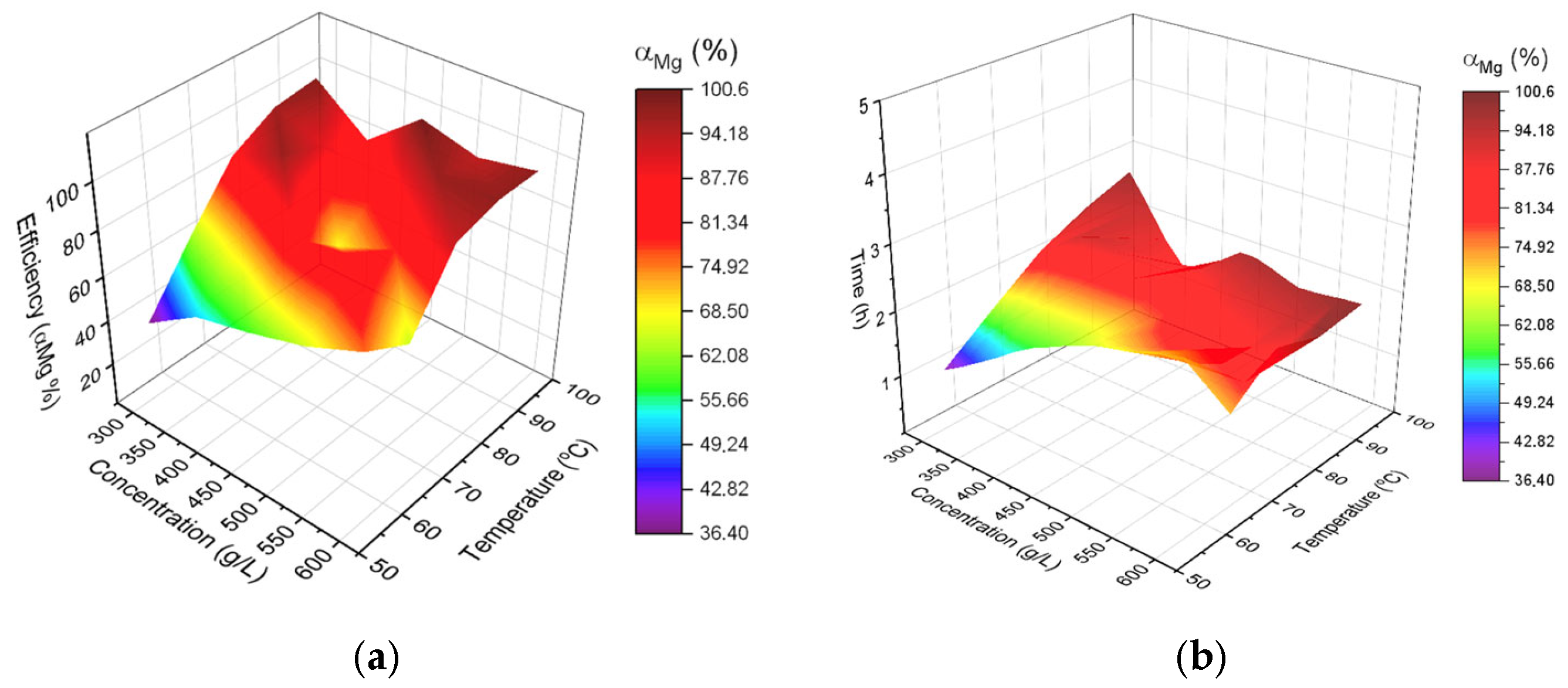
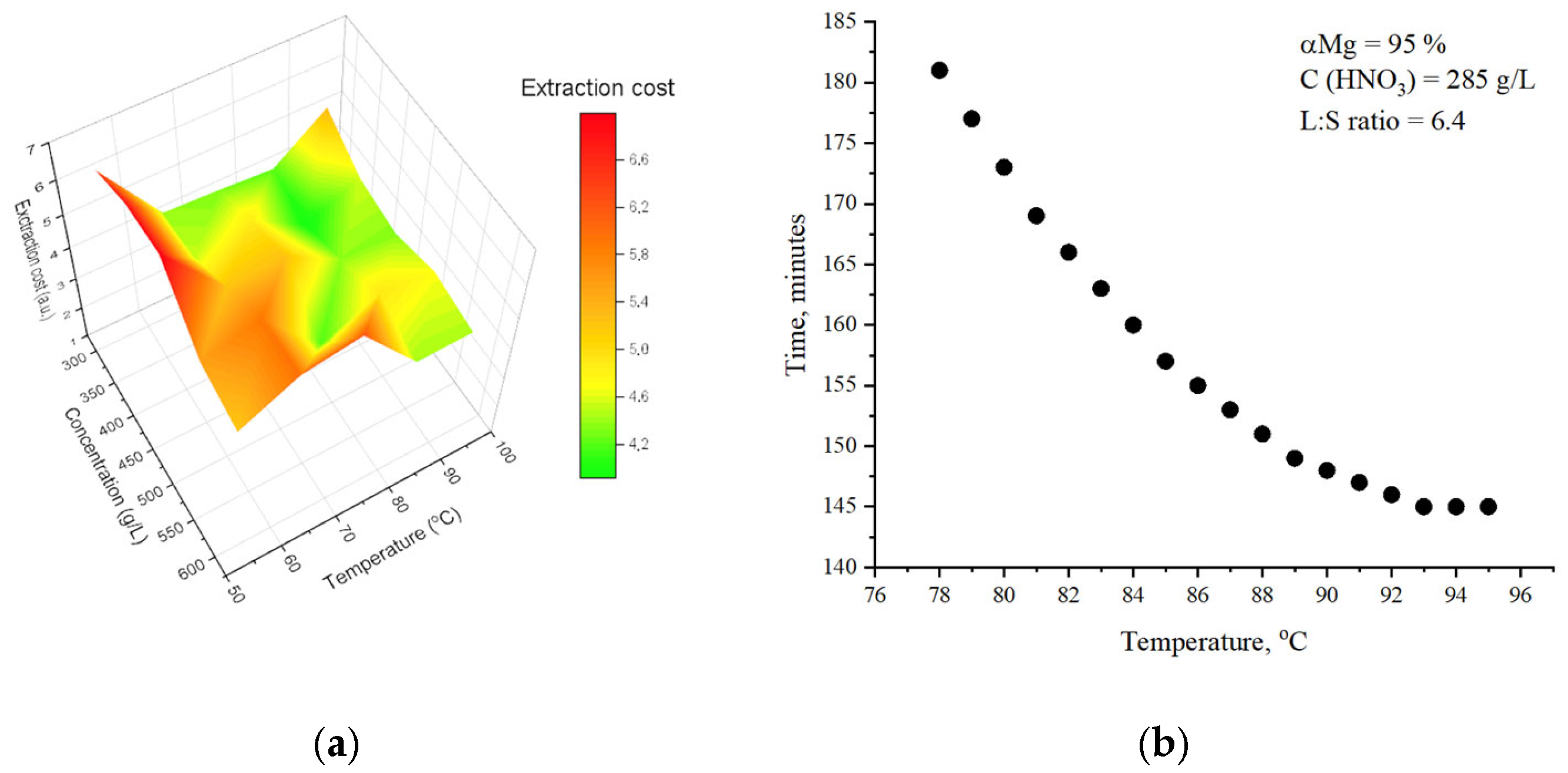
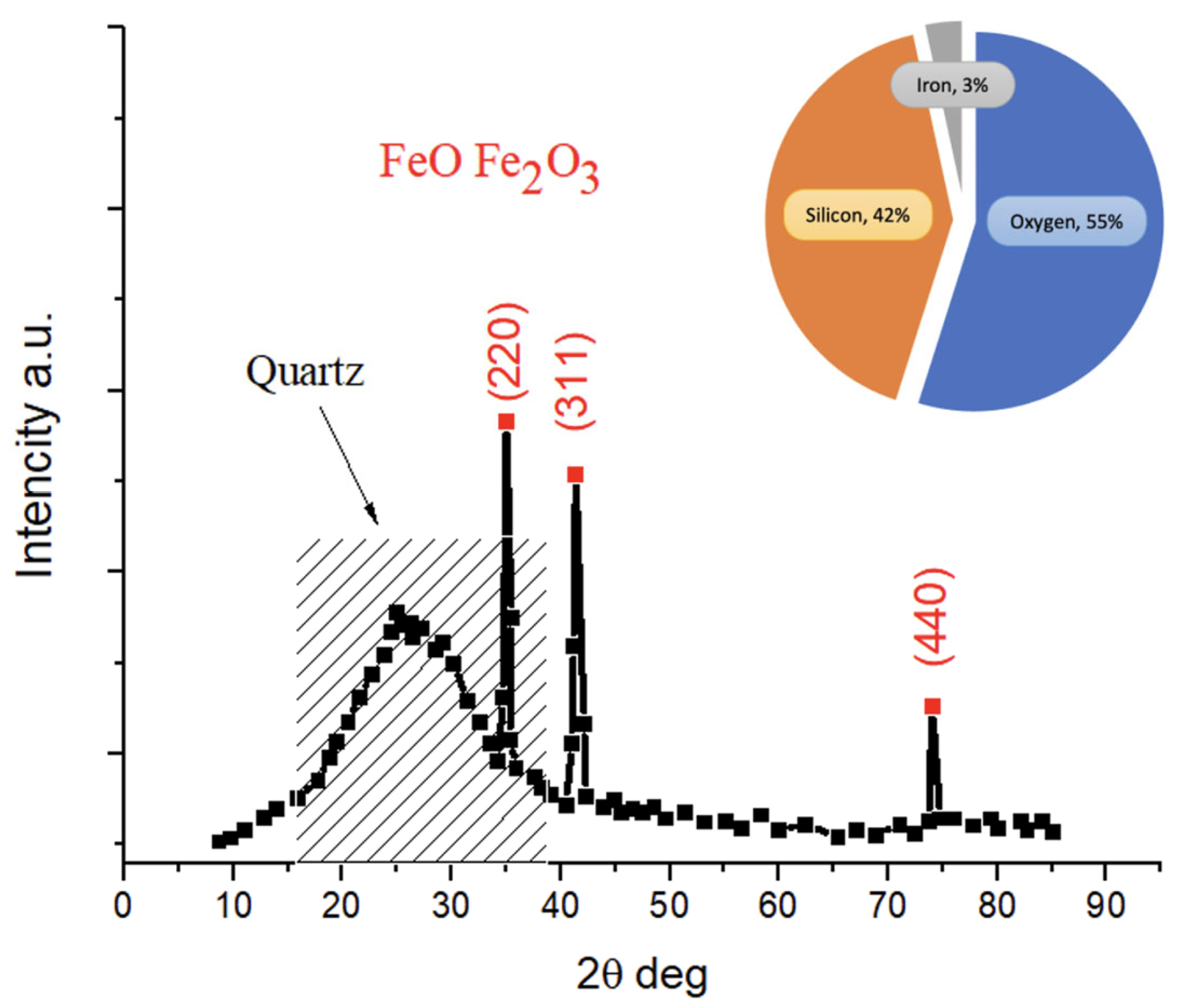
2. Results
3. Materials and Methods
3.1. Materials
3.2. Metods
3.2.1. Acid Leaching
3.2.2. Design of Experiments
3.2.3. Model Description
3.2.4. Calculation of the Cost Efficiency
3.2.5. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| № | AC (g/L) | L:S (Liquid-Solid) | T (°C) | t (min) | PS (um) |
|---|---|---|---|---|---|
| 1 | 300 | 3:1 | 55 | 60 | 71 |
| 2 | 300 | 5:1 | 75 | 120 | 315 |
| 3 | 300 | 4:1 | 65 | 90 | 140 |
| 4 | 300 | 7:1 | 95 | 180 | 1250 |
| 5 | 300 | 6:1 | 85 | 150 | 630 |
| 6 | 450 | 3:1 | 75 | 90 | 630 |
| 7 | 450 | 5:1 | 65 | 180 | 71 |
| 8 | 450 | 4:1 | 95 | 150 | 315 |
| 9 | 450 | 7:1 | 85 | 60 | 140 |
| 10 | 450 | 6:1 | 55 | 120 | 1250 |
| 11 | 375 | 3:1 | 65 | 150 | 1250 |
| 12 | 375 | 5:1 | 95 | 60 | 630 |
| 13 | 375 | 4:1 | 85 | 120 | 710 |
| 14 | 375 | 7:1 | 55 | 90 | 315 |
| 15 | 375 | 6:1 | 75 | 180 | 140 |
| 16 | 600 | 3:1 | 95 | 120 | 140 |
| 17 | 600 | 5:1 | 85 | 90 | 1250 |
| 18 | 600 | 4:1 | 55 | 180 | 630 |
| 19 | 600 | 7:1 | 75 | 150 | 71 |
| 20 | 600 | 6:1 | 65 | 60 | 315 |
| 21 | 525 | 3:1 | 85 | 180 | 315 |
| 22 | 525 | 5:1 | 55 | 150 | 140 |
| 23 | 525 | 4:1 | 75 | 60 | 1250 |
| 24 | 525 | 7:1 | 65 | 120 | 630 |
| 25 | 525 | 6:1 | 95 | 90 | 71 |
A.1.1. Analysis of Particle Size as a Non-Significant Factor in the Model
| Function | R | tr | Function Significance |
|---|---|---|---|
| Y1 | 0.96 | 20.02 > 2 | Significant |
| Y2 | 0.99 | 90.31 > 2 | Significant |
| Y3 | 1.00 | 1116.59 > 2 | Significant |
| Y4 | 0.97 | 30.80 > 2 | Significant |
| Y5 | 0.07 | 0.11 < 2 | Not significant |
A.1.2. Model Validation and Error Analysis
| № | |||
|---|---|---|---|
| 1 | 36.47 | 43.15 | 6.68 |
| 2 | 85.87 | 81.61 | 4.26 |
| 3 | 62.32 | 65.16 | 2.84 |
| 4 | 98.70 | 102.77 | 4.07 |
| 5 | 96.23 | 94.27 | 1.96 |
| 6 | 68.67 | 73.43 | 4.76 |
| 7 | 84.19 | 85.57 | 1.38 |
| 8 | 100.42 | 102.15 | 1.73 |
| 9 | 83.03 | 81.97 | 1.06 |
| 10 | 58.08 | 59.17 | 1.09 |
| 11 | 71.69 | 70.22 | 1.47 |
| 12 | 81.04 | 81.23 | 0.19 |
| 13 | 89.35 | 90.31 | 0.96 |
| 14 | 52.06 | 53.44 | 1.38 |
| 15 | 96.64 | 95.83 | 0.81 |
| 16 | 97.95 | 92.98 | 4.97 |
| 17 | 97.84 | 93.87 | 3.97 |
| 18 | 76.19 | 70.51 | 5.68 |
| 19 | 92.91 | 99.33 | 6.42 |
| 20 | 64.92 | 69.84 | 4.92 |
| 21 | 96.56 | 99.55 | 2.99 |
| 22 | 65.40 | 65.07 | 0.33 |
| 23 | 78.76 | 77.26 | 1.50 |
| 24 | 87.55 | 78.11 | 9.44 |
| 25 | 93.46 | 93.49 | 0.03 |
References
- Shayakhmetova, R.A.; Mukhametzhanova, A.A.; Akbayeva, D.N.; Terlikbaeva, A.Z.; Osipov, P.A.; Alimzhanova, A.M.; Zharmenov, A.A. Magnesium and silicon recovery from chrysotile asbestos waste of the deposit Zhitikara, Kazakhstan. Sci. Rep. 2024, 14, 31866. [Google Scholar] [CrossRef] [PubMed]
- Akylbekov, Y.Y.; Shevko, V.M.; Aitkulov, D.K.; Karataeva, G.E. Electrothermal processing of chrysotile-asbestos wastes with production of ferroalloy and extraction of magnesium into the gas phase. Complex Use Miner. Resour. 2023, 327, 74–81. [Google Scholar] [CrossRef]
- Chu, L.; Sun, H.; Peng, T.; Lu, H.; Li, M.; Zhang, Y.; Luo, L. Selective extraction of Mg2+ from chrysotile asbestos tailings via ammonium sulfate roasting and water leaching: Process optimization and mechanistic insights. J. Clean. Prod. 2025, 515, 145773. [Google Scholar] [CrossRef]
- Girotto, C.P.; de Campos, S.D.; de Campos, É.A. Chrysotile asbestos treated with phosphoric acid as an adsorbent for ammonia nitrogen. Heliyon 2020, 6, e03397. [Google Scholar] [CrossRef]
- Li, G.; Yang, Y.; Li, B.; Zhang, X.; Xie, W.; Wei, G.; Yang, Y.; Peng, X.; Tan, J. Innovative developments and strategic utilization of advanced magnesium alloys in aerospace engineering. J. Alloys Compd. 2025, 1039, 183073. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Nghiem, L.D.; Hai, F.I. Extraction of strategically important elements from brines: Constraints and opportunities. Water Res. 2020, 168, 115149. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of magnesium and its alloys: A review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Devi, K.R.; Sreekanth, D. Novel multicomponent magnesium alloys: High strength-elongation synergy and PEO-driven corrosion resistance. Results Eng. 2025, 26, 105193. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhari, A.; Gupta, A.K.; Kumar, A. Rare-Earth based magnesium alloys as a potential biomaterial for the future. J. Magnes. Alloys 2024, 12, 3841–3897. [Google Scholar] [CrossRef]
- Gohar, O.; Ishfaq, H.A.; Iqbal, M.A.; Khan, M.Z.; Basharat, S.; Kanwal, N.; Riaz, A.; Saleem, M.; Koh, J.-H.; Hussain, I.; et al. Recent advancements in high-performance and durable electrodes materials for magnesium-ion batteries. Coord. Chem. Rev. 2025, 538, 216702. [Google Scholar] [CrossRef]
- Mizanuzzaman, M.; Chowdhury, M.A.; Khandaker, T.; Islam, M.S.; Kowser, M.A.; Islam, M.M. Advancements in cathode materials for high-performance rechargeable magnesium-ion batteries. J. Energy Storage 2025, 118, 116213. [Google Scholar] [CrossRef]
- Güllü, Ö.; Türkeri, M.; Tataroğlu, A.D.E.M. Electrical and optical characterization of Al/MgO/p-Si (MIS) diode with magnesium oxide thin films by spraying method. Solid State Commun. 2025, 403, 115992. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Wu, Z.; Wang, W.; Sun, K.; Wang, C.; Wang, C.; Cao, M.; Fu, J. Magnesium Oxide Nanoparticle-Enhanced Bioactive 3D-Printed Denture Resins: Optimized Antifungal Performance and Improved Clinical Applicability. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137992. [Google Scholar] [CrossRef]
- Shalomeev, V.A.; Greshta, V.L.; Papirov, I.I.; Shokurov, V.S.; Pikalov, A.I.; Mukhachev, A.P.; Yelatontsev, D.O. Advances and prospects of high-purity magnesium and its alloys in medicine–a concise review. J. Alloys Compd. Commun. 2024, 3, 100011. [Google Scholar] [CrossRef]
- Zhi, Y.; Mei, B.; Li, W.; Cheng, J.; Luo, P. Synthesis of highly sinterable MgO powders for IR transparent ceramics. Ceram. Int. 2025, 51, 26696–26701. [Google Scholar] [CrossRef]
- Collado, S.; Oulego, P.; Vázquez, S.; Pola, L.; Díaz, M. Characterization and reuse of waste from the magnesium nitrate fertilizer industry. Sci. Total Environ. 2023, 877, 162925. [Google Scholar] [CrossRef]
- Song, Y.; Han, Z.; Xie, Y.; Zhong, H.; He, Z. Mechanism of oxidation enhanced extraction rubidium from biotite using magnesium nitrate in a sulfuric acid leaching process. Process Saf. Environ. Prot. 2025, 201, 107538. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Association of hypomagnesemia with cardiovascular diseases and hypertension. Int. J. Cardiol. Hypertens. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Pardo, M.R.; Vilar, E.G.; Martín, I.S.M.; Martín, M.A.C. Bioavailability of magnesium food supplements: A systematic review. Nutrition 2021, 89, 111294. [Google Scholar] [CrossRef]
- Available online: https://market.us/report/global-magnesium-oxide-market/table-of-content/ (accessed on 11 November 2025).
- Royani, A.; Sulistiyono, E.; Prasetiyo, A.B.; Subagja, R. Extraction of magnesium from calcined dolomite ore using hydrochloric acid leaching. AIP Conf. Proc. 2018, 1964, 020017. [Google Scholar] [CrossRef]
- Ballou, B.J.; Hanssen, K.O. A Method for Isolation and Production of Magnesium Metal, Magnesium Chloride, Magnesite and Magnesium Based Products. International Application Published Under the Patent Cooperation Treaty (PCT). International Application Number: PCT/NO98/241, 8 June 1998. [Google Scholar]
- Hoşgün, H.L.; Kurama, H. Dissolution kinetics of magnesite waste in HCl solution. Ind. Eng. Chem. Res. 2012, 51, 1087–1092. [Google Scholar] [CrossRef]
- Ash, S. Mineral Commodity Summaries 2019; US Geological Survey: Reston, VA, USA, 2019. [CrossRef]
- van Zandwijk, N.; Frank, A.L.; Reid, G.; Røe, O.D.; Amos, C.I. Asbestos-Related lung Cancer: An underappreciated oncological issue. Lung Cancer 2024, 194, 107861. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- El-Sayed, D.; Ismail, A.K.; El-Hosiny, F.I. Magnesium Chloride Crystals with Studying Mechanism and Leaching Kinetics of Serpentinite Ore by Hydrochloric Acid. Trans. Indian Inst. Met. 2023, 76, 1439–1446. [Google Scholar] [CrossRef]
- McCutcheon, J.; Dipple, G.M.; Wilson, S.A.; Southam, G. Production of magnesium-rich solutions by acid leaching of chrysotile: A precursor to field-scale deployment of microbially enabled carbonate mineral precipitation. Chem. Geol. 2015, 413, 119–131. [Google Scholar] [CrossRef]
- Morgan, A. Acid leaching studies of chrysotile asbestos from mines in the Coalinga region of California and from Quebec and British Columbia. Ann. Occup. Hyg. 1997, 41, 249–268. [Google Scholar] [CrossRef]
- Konlechner, D.; Kappacher, G. Achieving the Carbon-Neutral Production of Magnesia and Silica Products Using a HCl-Based Process in Serpentine Feedstock. Mater. Proc. 2021, 5, 19. [Google Scholar] [CrossRef]
- Zhang, M.H.; Zhao, L.; Xu, H.L.; Wu, W.C.; Dong, H. Study on the thermal decomposition mechanism of Mg(NO3)2·6H2O from the perspective of resource utilization of magnesium slag. Environ. Technol. 2024, 45, 751–761. [Google Scholar] [CrossRef]
- He, F.; Ma, B.; Qiu, Z.; Wang, C.; Chen, Y.; Hu, X. Enhanced extraction of nickel from limonitic laterite via improved nitric acid pressure leaching process. Miner. Eng. 2023, 201, 108170. [Google Scholar] [CrossRef]
- Geymond, U.; Loiseau, K.; Roche, V.; Pasquet, G.; Revillon, S.; Sougrati, M.; Moretti, I. Mössbauer spectroscopy: A key tool to quantify Fe-speciation and distribution in H2-generating rocks. Appl. Geochem. 2025, 187, 106399. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Evans, B.W.; Kuehner, S.M. Serpentine–Hisingerite Solid Solution in Altered Ferroan Peridotite and Olivine Gabbro. Minerals 2019, 9, 47. [Google Scholar] [CrossRef]
- Valouma, A.; Verganelaki, A.; Tetoros, I.; Maravelaki-Kalaitzaki, P.; Gidarakos, E. Magnesium oxide production from chrysotile asbestos detoxification with oxalic acid treatment. J. Hazard. Mater. 2017, 336, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yeskibayeva, C.; Auyeshov, A.; Arynov, K.; Dikanbayeva, A.; Satimbekova, A. Nature of serpentinite interactions with low-concentration sulfuric acid solutions. Green Process. Synth. 2024, 13, 20240034. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rashid, M.I.; Khan, S.A.; Waleed, M.; Shahzad, M.U. Magnesium extraction from serpentine for carbon capture and storage. J. Pak. Inst. Chem. Eng. 2025, 52, 51–57. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, B.; Wang, C.; Zhang, Y.; Shi, S.; Chen, Y. Investigation of the thermal behavior of Mg(NO3)2·6H2O and its application for the regeneration of HNO3 and MgO. Chem. Eng. J. 2022, 433, 133804. [Google Scholar] [CrossRef]
- Taheri, B.; Larachi, F. Mineral-Based Magnesium Extraction Technologies: Current and Future Practices. Processes 2025, 13, 2945. [Google Scholar] [CrossRef]
- Yang, J.; Duan, X.; Liu, L.; Yang, H.; Jiang, X. Recovery of magnesium from ferronickel slag to prepare magnesium oxide by sulfuric acid leaching. Minerals 2021, 11, 1375. [Google Scholar] [CrossRef]
- Troeglazova, A.V. Probability-determined design planning for decomposition of silicon-containing sample. Interexpo GEO-Sib. 2020, 2, 49–55. [Google Scholar] [CrossRef]
- Available online: https://www.assda.asn.au/publications/technical-faqs/faq-8-general-corrosion-resistance (accessed on 11 November 2025).
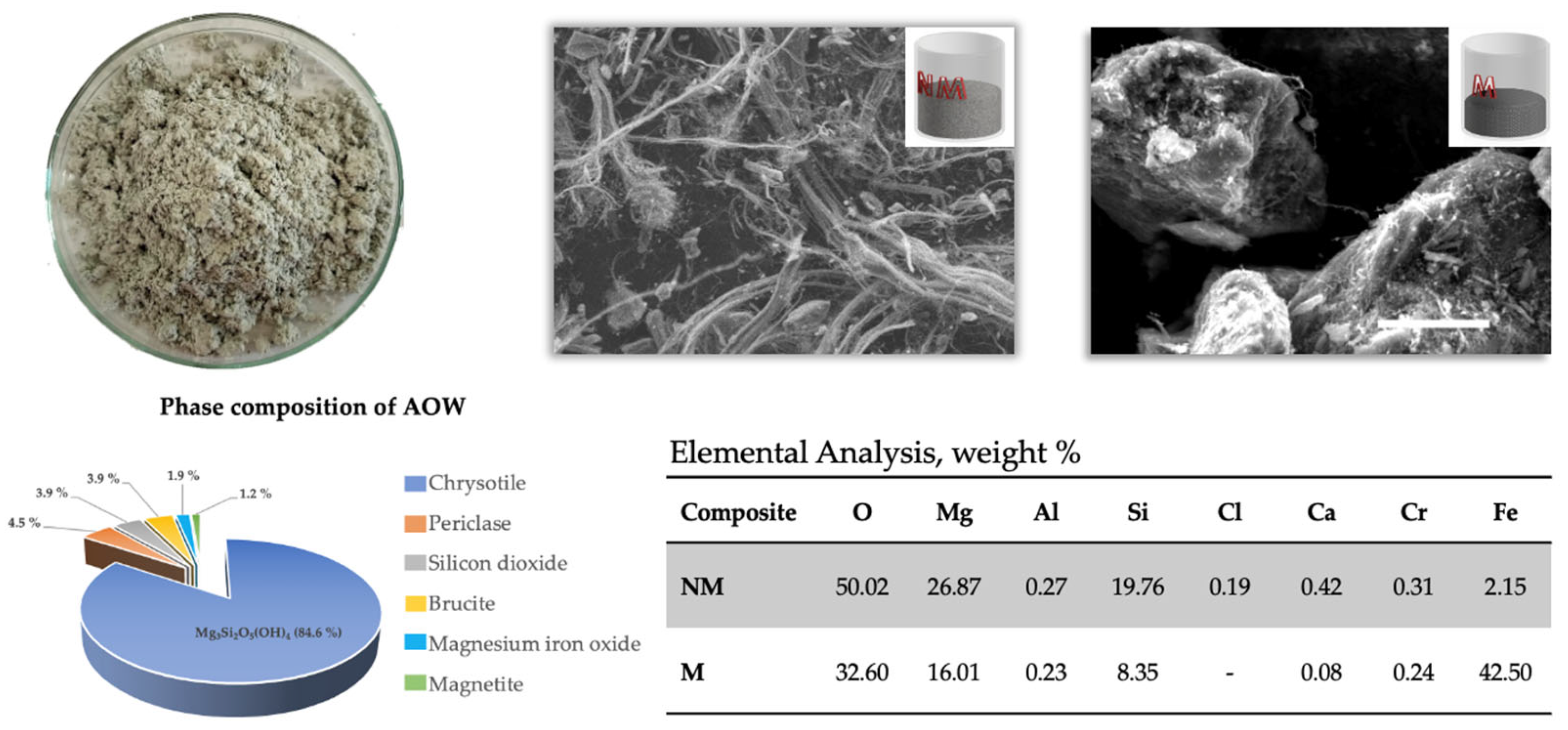
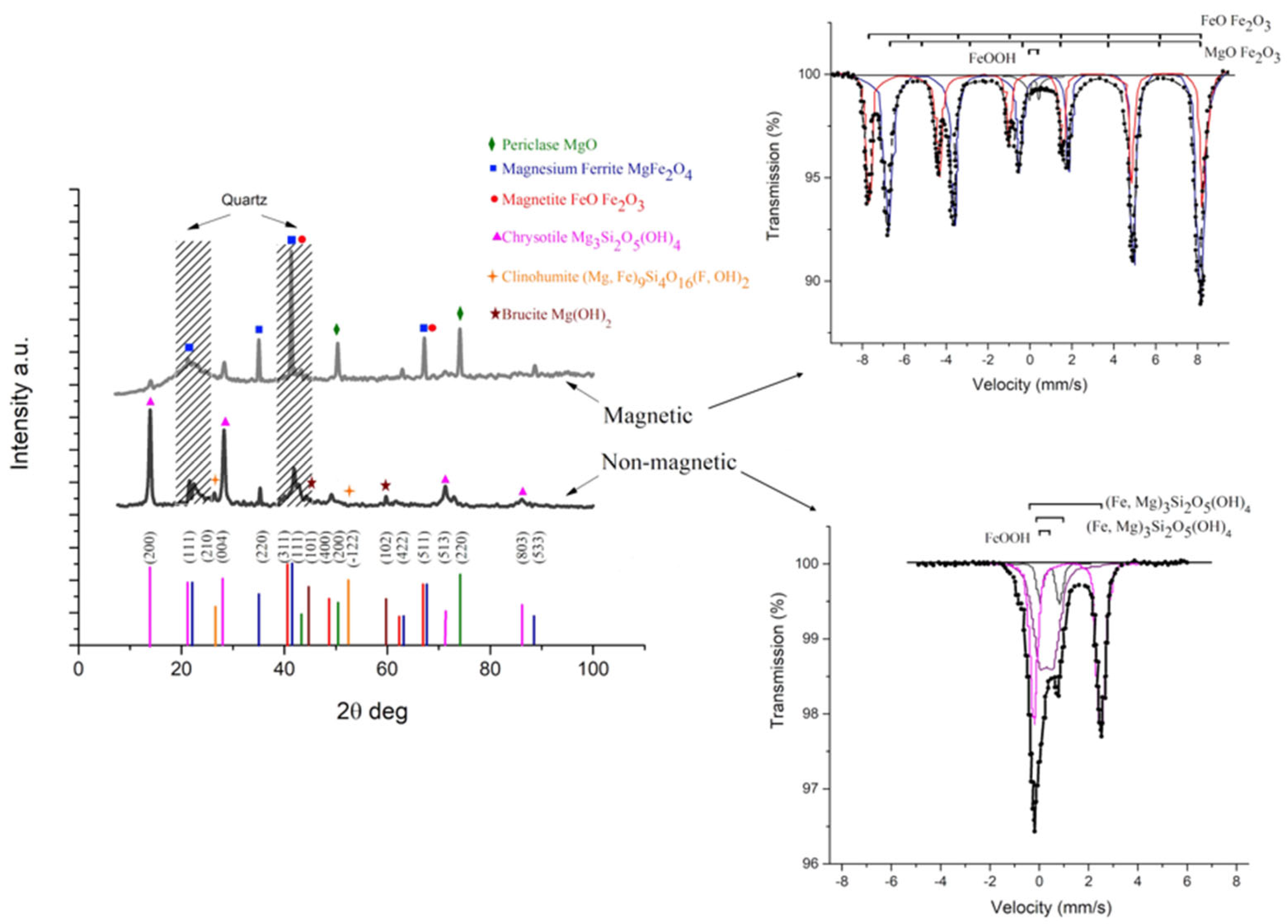
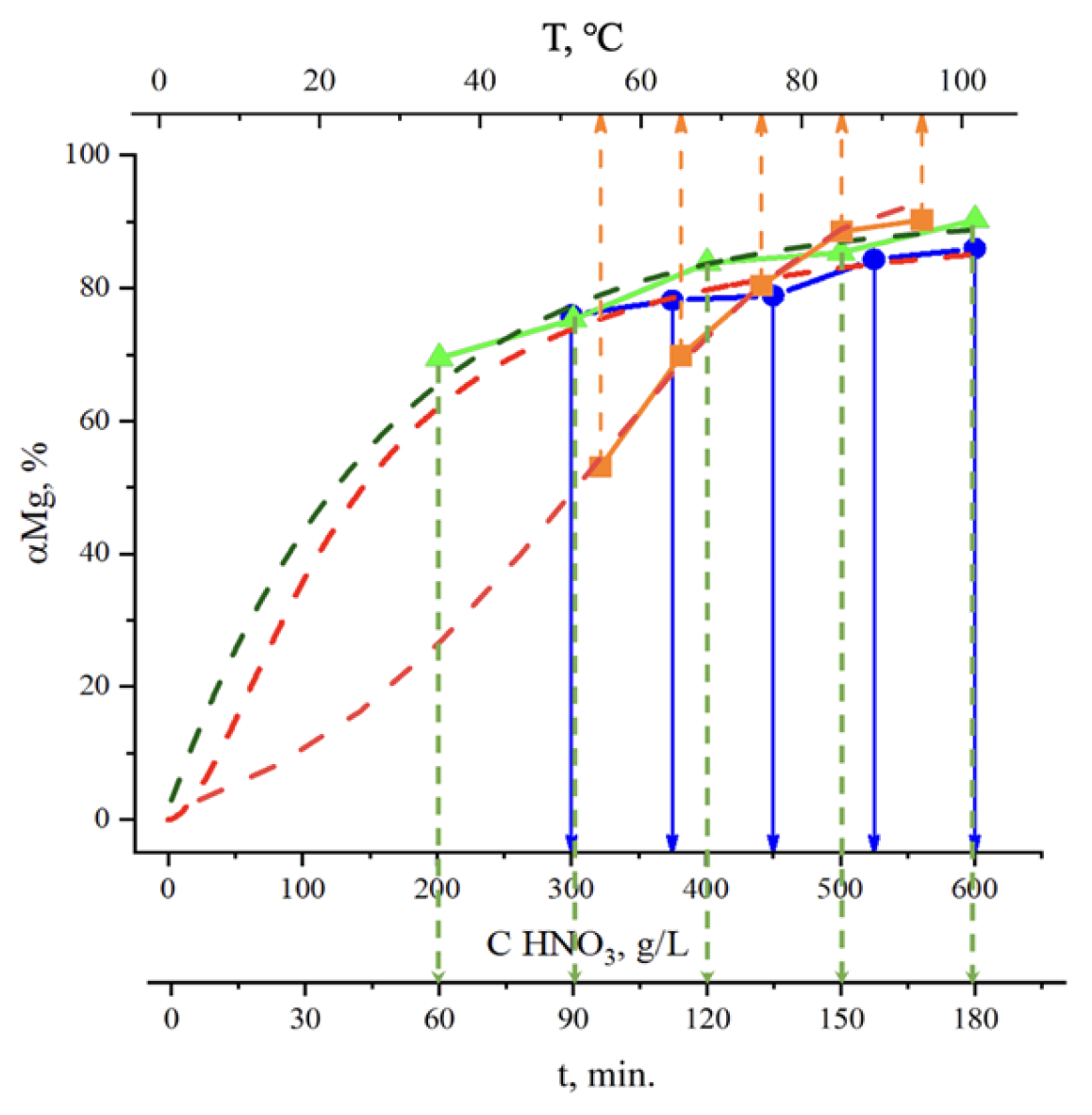
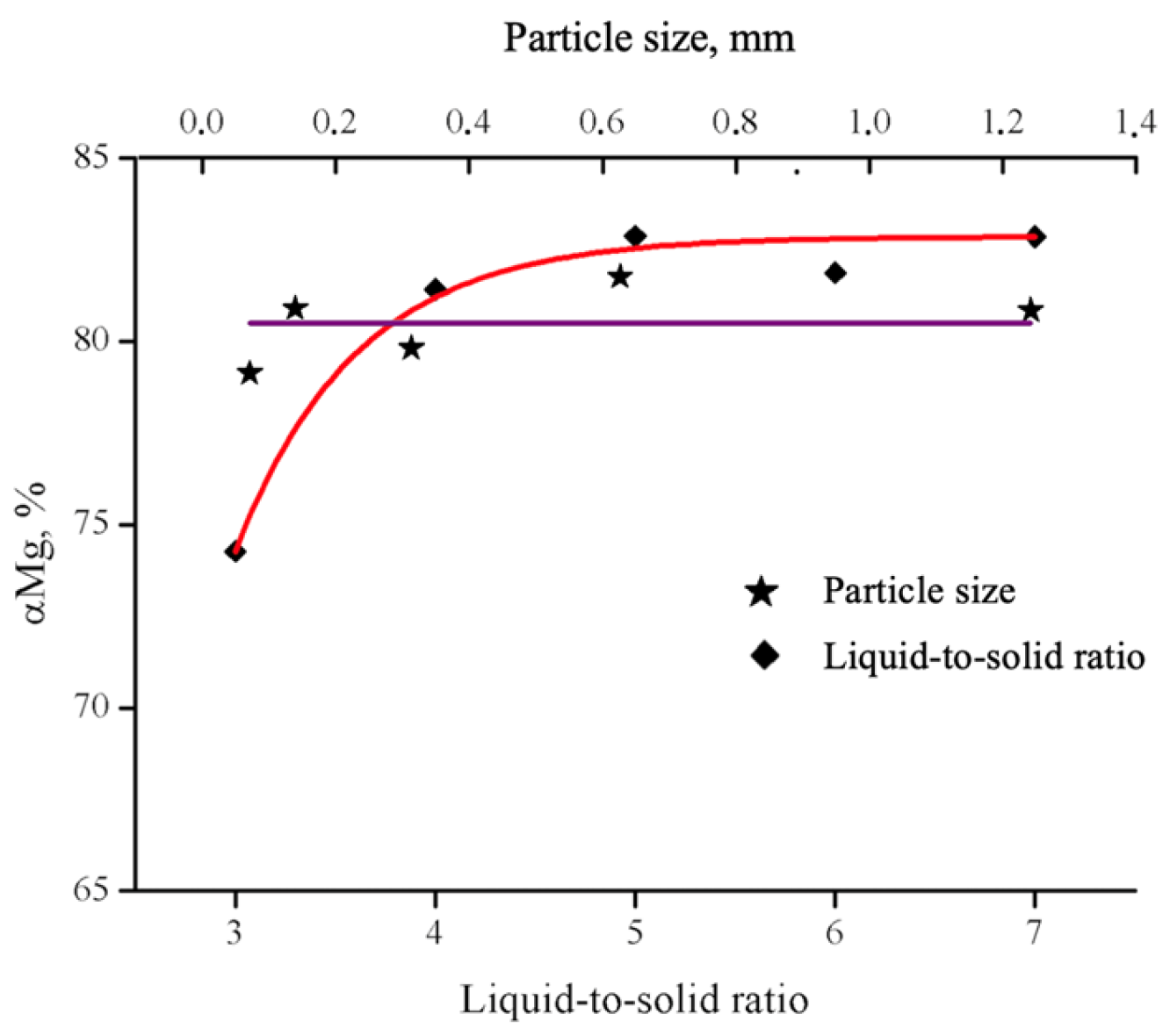

| № | Leaching Agent | Conditions | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| H2SO4 | Serpentinite; C (H2SO4) = 10–60%; T = 90 °C; S/L = 1:10; t = 2 h. | Mg yield 85–90%; Low corrosive activity of the medium; Closed-cycle process. | Difficult purification and production of MgO; High decomposition temperature (700 °C); Difficult filtration of SiO2 precipitate. | [37] | |
| HCl | Chrysotile; C(HCl) = 2–6 M; T = 60–90 °C; t = 120 min. | Mg yield 89–92%; Production of pure MgO; Closed-cycle process; HCl regeneration; CO2-neutral technology. | High decomposition temperature (800 °C); High corrosive activity of HCl; Significant Fe3+ dissolution; Difficult filtration of SiO2 precipitate. | [28,30,31] | |
| (NH4)2SO4 | Chrysotile tailings; (NH4)2SO4: solid = 1:1.15; T = 700–800 °C; t = 2 h; Aqueous leaching stage 60 °C: S/L = 1:10. | Mg yield up to 95%; closed-cycle process. | High decomposition temperature (800 °C); Large-scale process; Difficult purification and production of MgO; Absence of a closed cycle. | [3] | |
| HNO3 | Chrysotile tailings; C(HNO3) = 450 g/L; T = 65–85 °C; t = 2–3 h. | Mg yield 90%; production of high-purity MgO; HNO3 regeneration; Moderate decomposition temperature (<500 °C); Closed-cycle process. | Formation of toxic nitrogen oxides (NO, NO2). | [present article] | |
| H2C2O4 | Chrysotile asbestos; C(H2C2O4) = 0.05 M; t = 8 days; T = 26 °C. | Eco-friendly reagents; Moderate decomposition temperature (480 °C). | Mg yield 8.3%; Low leaching rate; Difficult purification of MgC2O4. | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, N.S.; Kholkin, O.S.; Abilmagzhanov, A.Z.; Adelbayev, I.E.; Oparin, S.K.; Ivanova, N.; Kudryashov, V. Nitric Acid Leaching for Magnesium Extraction from Asbestos Ore Waste: From DoE to Predictive Modeling and Cost-Efficient Optimization. Molecules 2025, 30, 4396. https://doi.org/10.3390/molecules30224396
Ivanov NS, Kholkin OS, Abilmagzhanov AZ, Adelbayev IE, Oparin SK, Ivanova N, Kudryashov V. Nitric Acid Leaching for Magnesium Extraction from Asbestos Ore Waste: From DoE to Predictive Modeling and Cost-Efficient Optimization. Molecules. 2025; 30(22):4396. https://doi.org/10.3390/molecules30224396
Chicago/Turabian StyleIvanov, Nikolay S., Oleg S. Kholkin, Arlan Z. Abilmagzhanov, Iskander E. Adelbayev, Sergey K. Oparin, Nataliya Ivanova, and Vladislav Kudryashov. 2025. "Nitric Acid Leaching for Magnesium Extraction from Asbestos Ore Waste: From DoE to Predictive Modeling and Cost-Efficient Optimization" Molecules 30, no. 22: 4396. https://doi.org/10.3390/molecules30224396
APA StyleIvanov, N. S., Kholkin, O. S., Abilmagzhanov, A. Z., Adelbayev, I. E., Oparin, S. K., Ivanova, N., & Kudryashov, V. (2025). Nitric Acid Leaching for Magnesium Extraction from Asbestos Ore Waste: From DoE to Predictive Modeling and Cost-Efficient Optimization. Molecules, 30(22), 4396. https://doi.org/10.3390/molecules30224396








