Isosorbide as a Molecular Glass: New Insights into the Physicochemical Behavior of a Biobased Diol
Abstract
1. Introduction
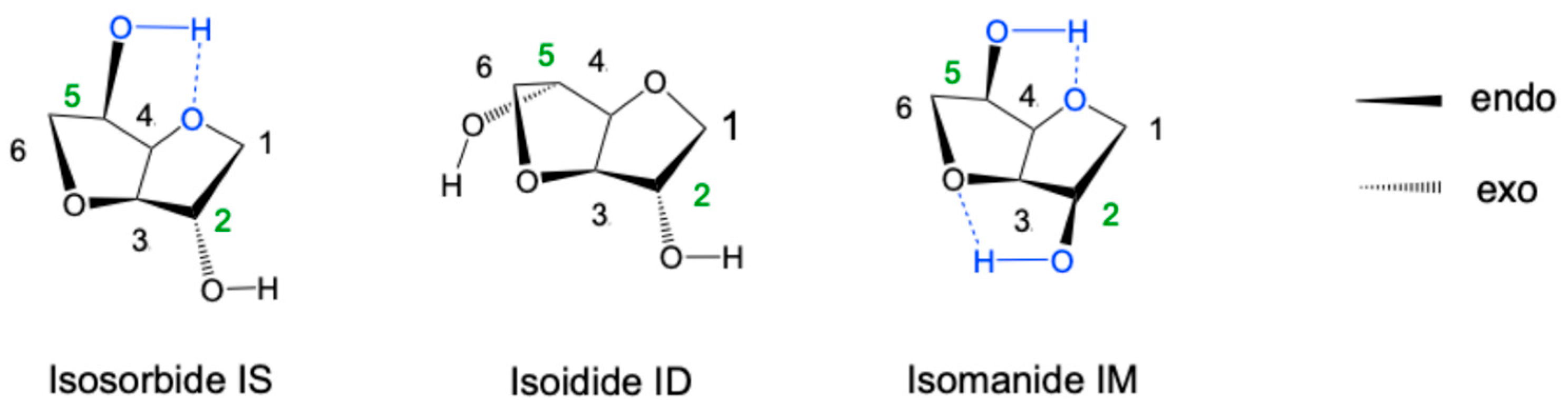
- Exo–exo or isoidide ID (or 1,4:3,6-dianhydro-L-iditol). In this arrangement, the hydroxyl hydrogens cannot form intramolecular hydrogen bonds with adjacent oxygens.
- Endo–endo or isomannide IM (or 1,4:3,6-dianhydro-D-mannitol). This symmetrical endo–endo conformation allows the formation of two intramolecular hydrogen bonds between the OH groups and the ether oxygens on each ring.
- Endo–exo or isosorbide IS (1,4:3,6-dianhydro-D-glucitol). One OH group is endo, allowing for a hydrogen bond as in IM, while the other is exo, making it more accessible to external reactive groups.
1.1. Isosorbide-Based Molecules for Therapeutic Applications
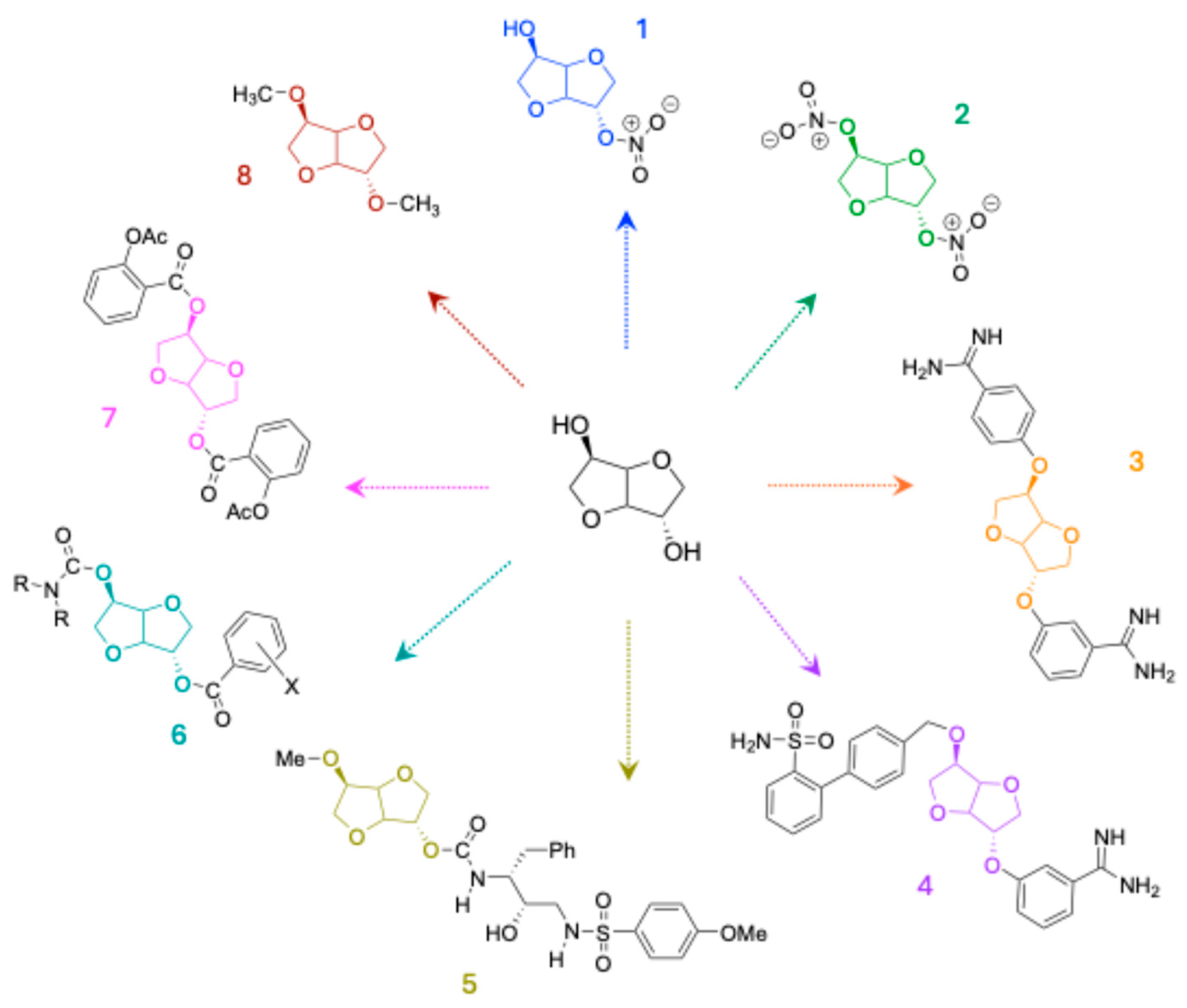
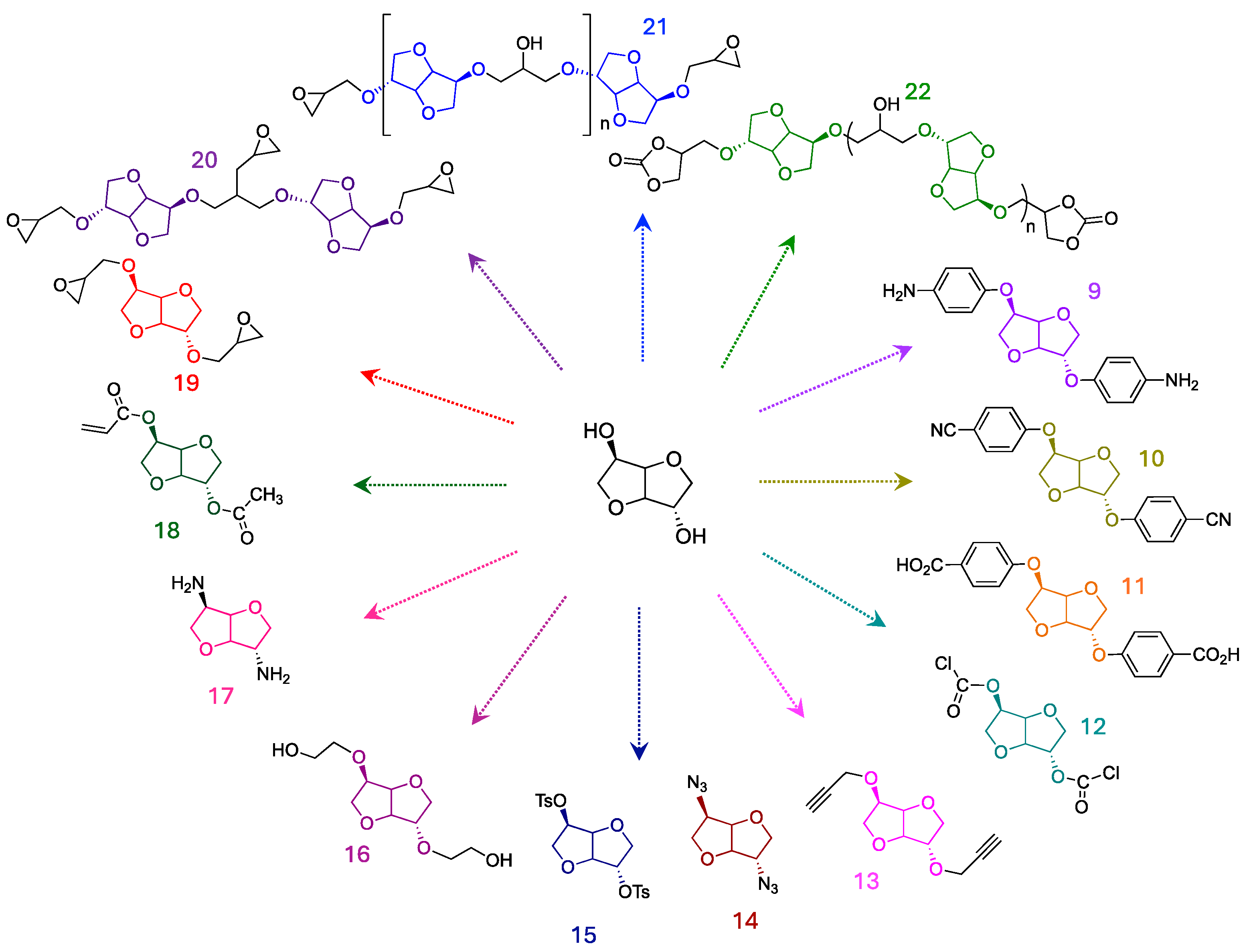
1.2. Monomers Generated from Isosorbide Functionalization
1.3. Polymers Properties Derived from Isosorbide
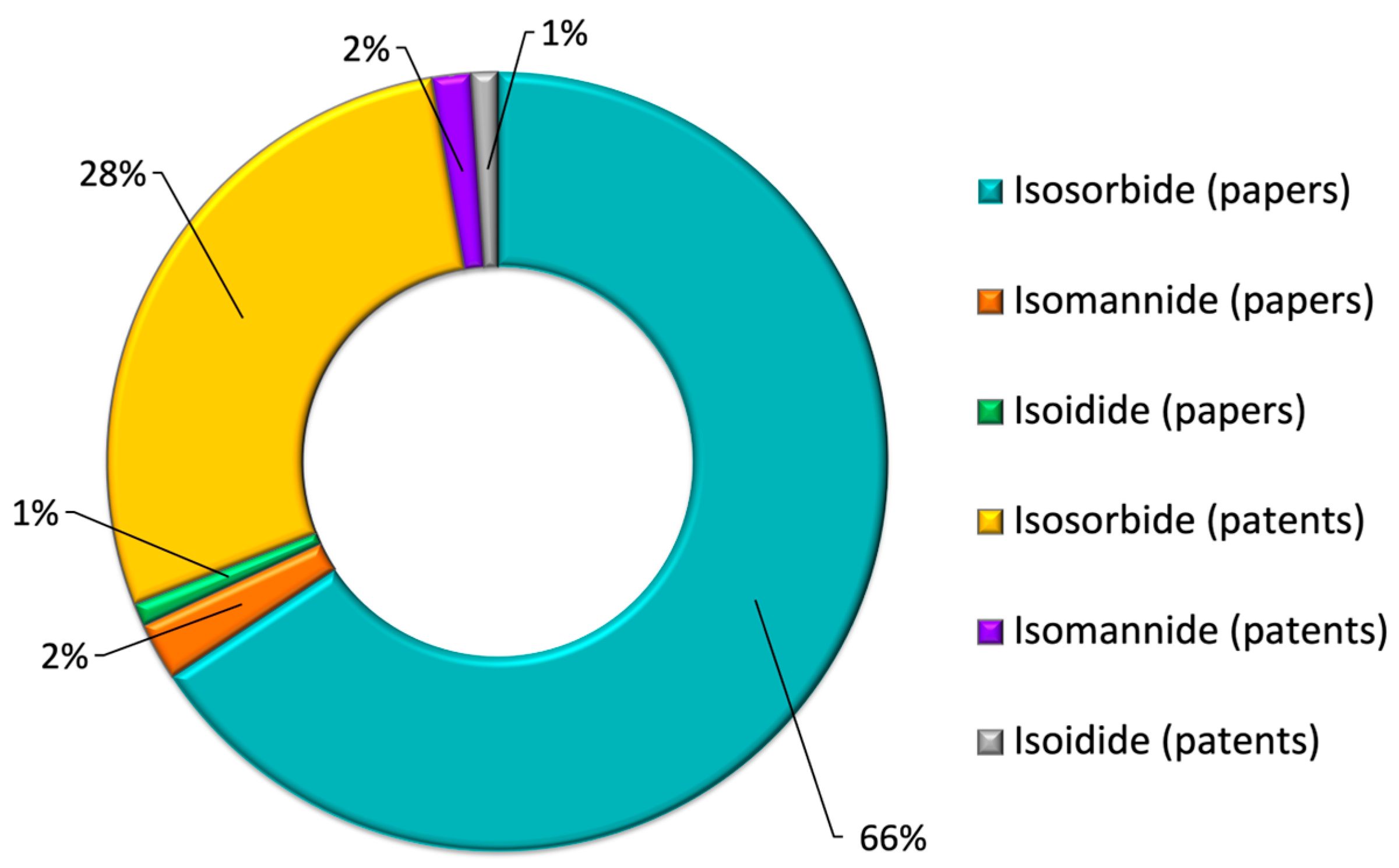
1.4. Motivations for Our Research
2. Results and Discussion
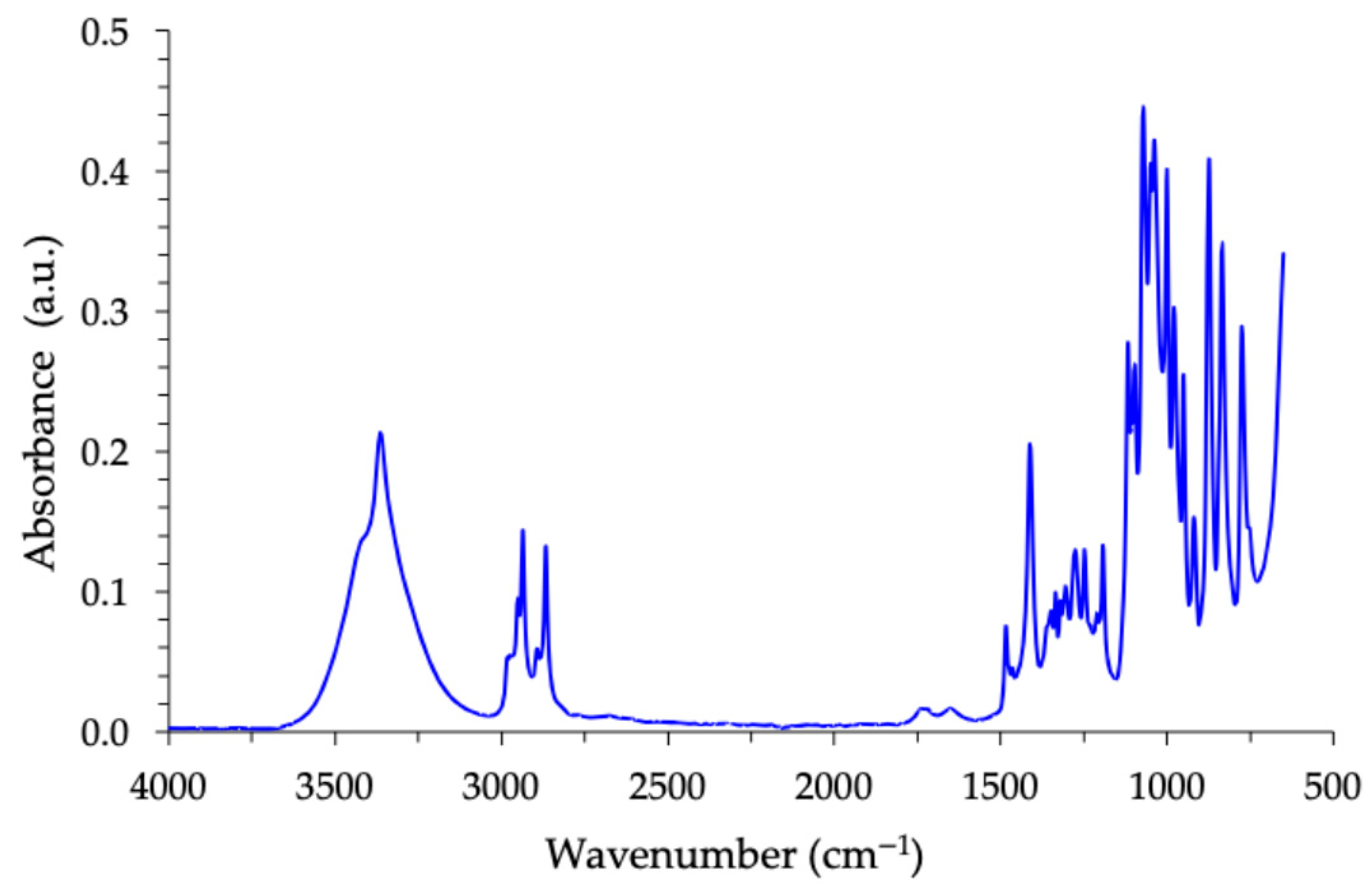
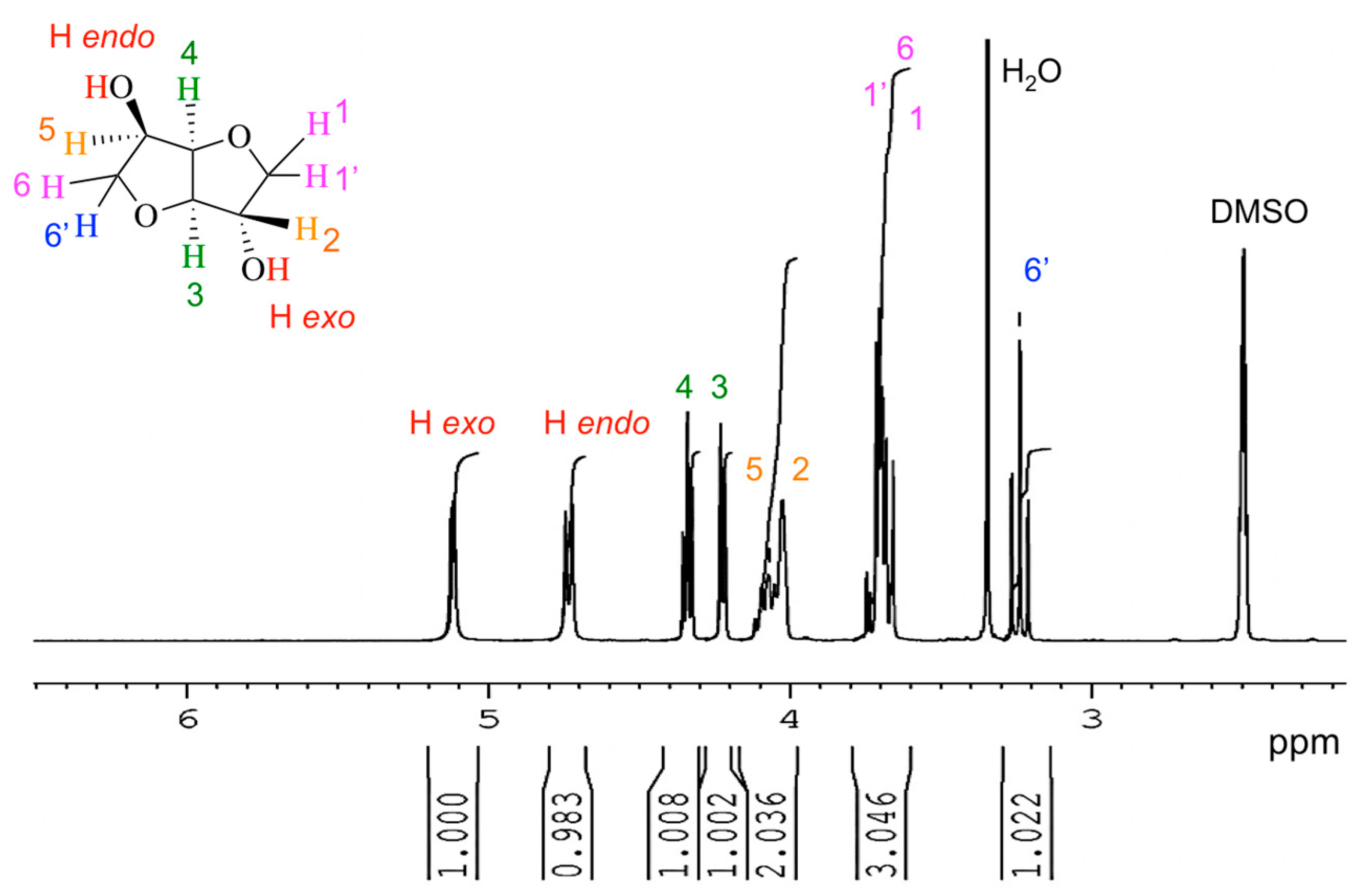
2.1. Chemical Analyses
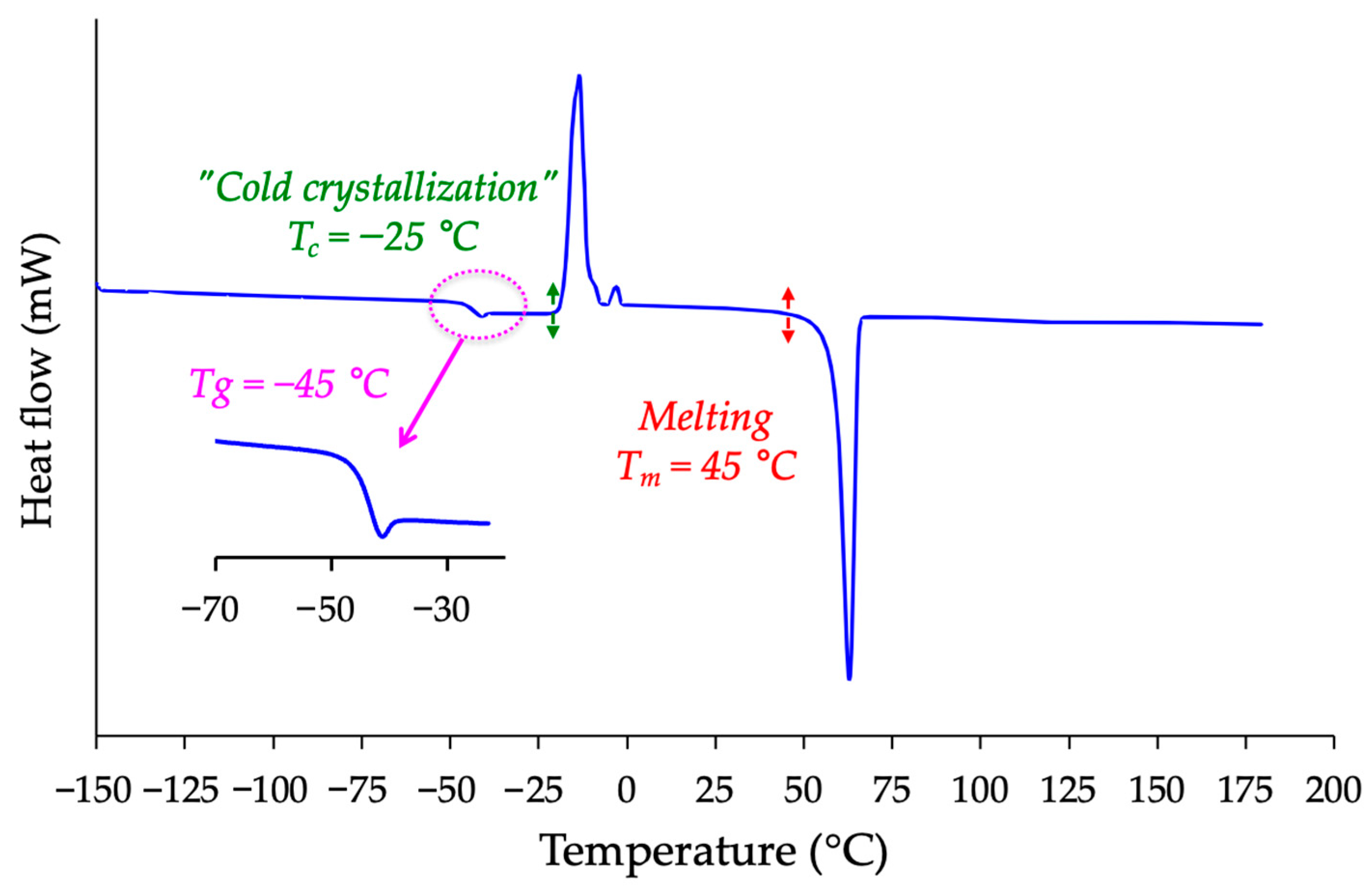
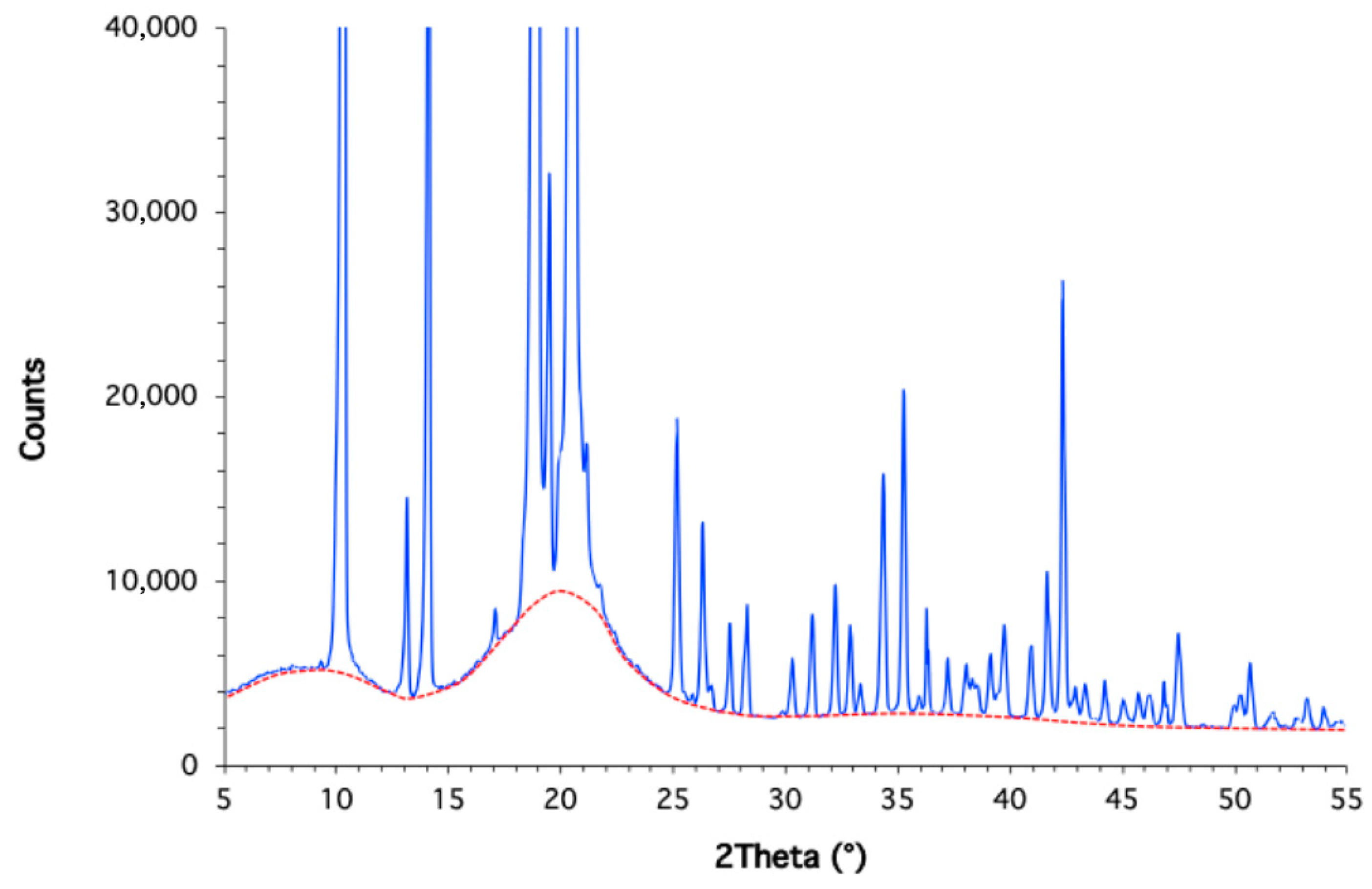
2.2. Differential Scanning Calorimetry (DSC) and X-Ray Diffraction (XRD)
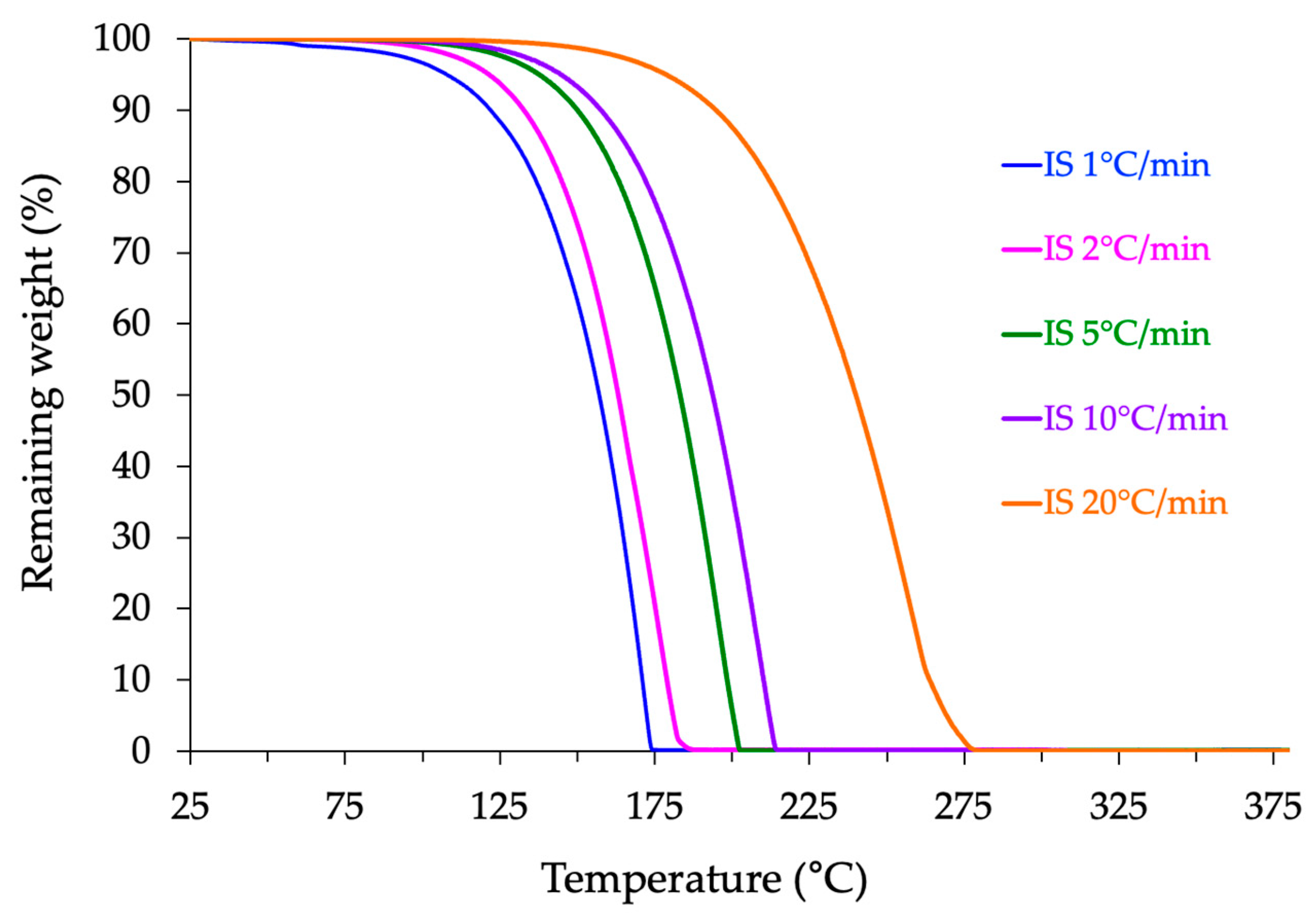
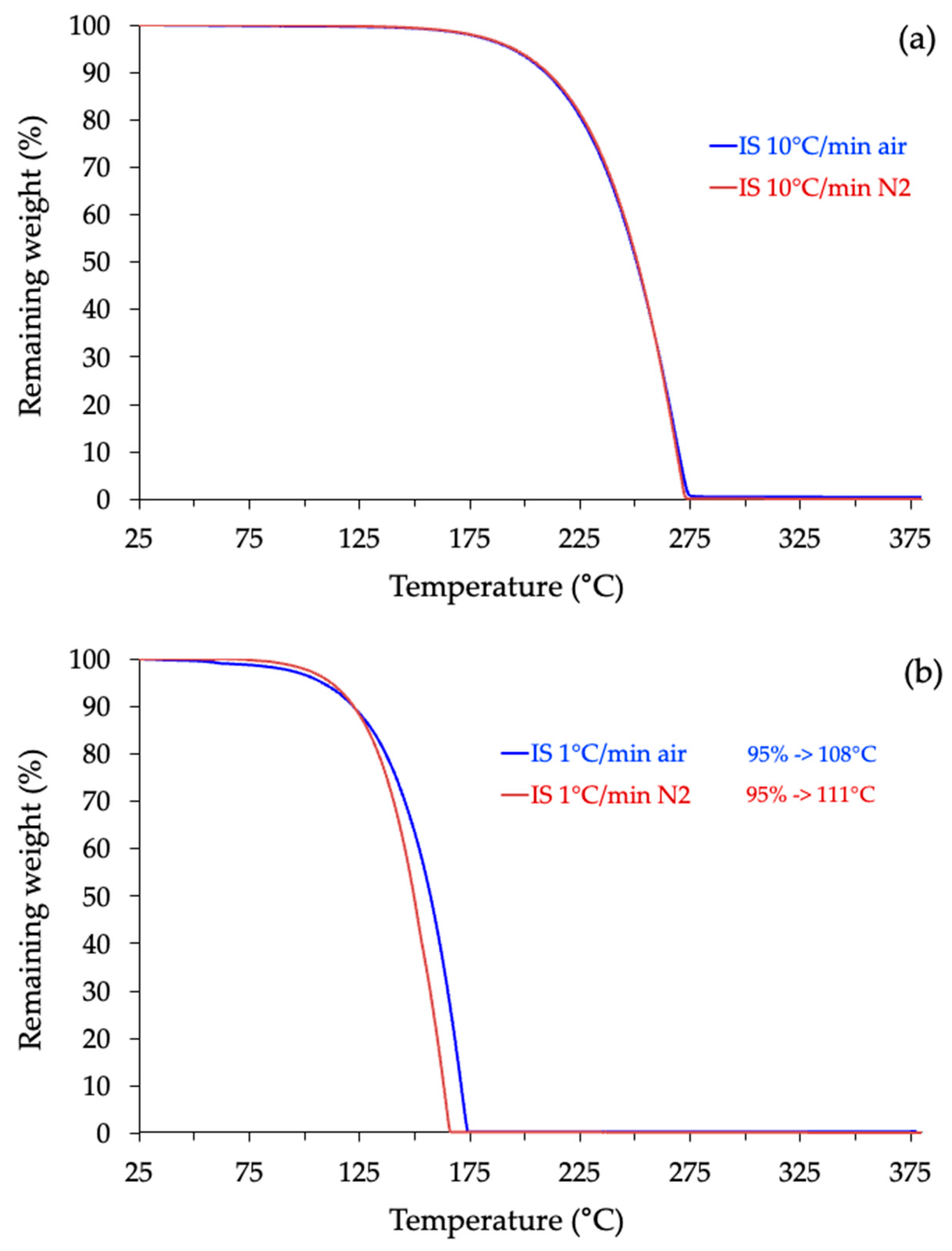
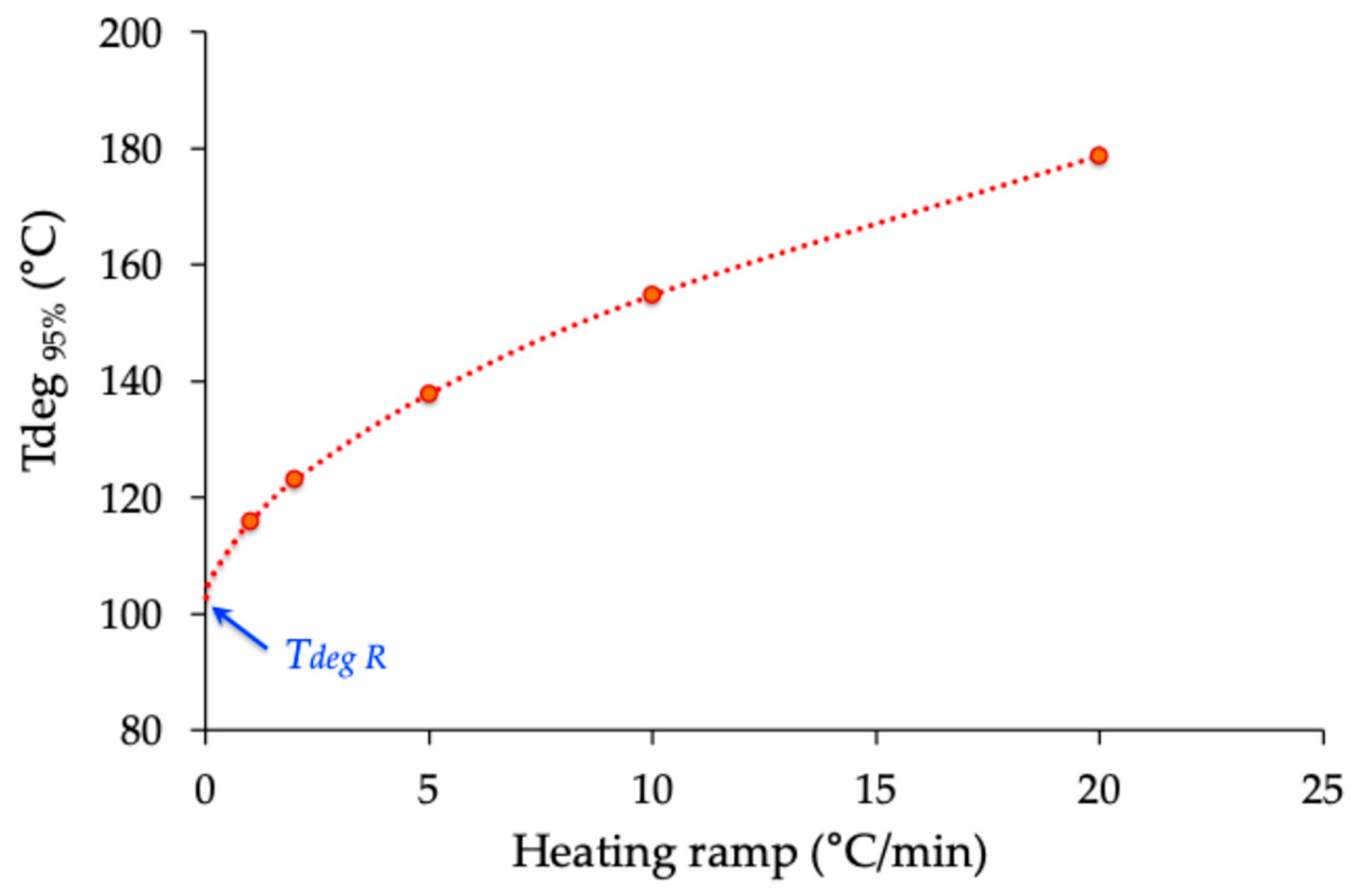
2.3. Thermogravimetric Analysis
2.4. Thermomechanical Properties
3. Materials and Methods
3.1. Isosorbide
3.2. Fourier Transform Infrared (FTIR) Experiment
3.3. Nuclear Magnetic Resonance (NMR) Analyses
3.4. Differential Scanning Calorimetry (DSC) Analyses
3.5. Thermogravimetric Analyses (TGA)
3.6. Thermomechanical Tests
3.7. X-Ray Diffraction Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleche, G.; Huchette, M. Isosorbide—Preparation, properties and chemistry. Starch-Starke 1986, 38, 26–30. [Google Scholar] [CrossRef]
- Hopton, F.J.; Thomas, G.H.S. Conformations of some dianhydrohexitols. Can. J. Chem. 1969, 47, 2395–2401. [Google Scholar] [CrossRef]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; Noordover, B.A.J.; van Es, D.S.; Koning, C.E. Semi-Aromatic Polyesters Based on a Carbohydrate-Derived Rigid Diol for Engineering Plastics. Chemsuschem 2015, 8, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Loupy, A.; Monteux, D. Asymmetric Diels-Alder: Monobenzylated isosorbide and isomannide as highly effective chiral auxiliaries. Tetrahedron Lett. 1996, 37, 7023–7026. [Google Scholar] [CrossRef]
- Noordover, B.A.J.; Haveman, D.; Duchateau, R.; van Benthem, R.A.T.M.; Koning, C.E. Chemistry, Functionality, and Coating Performance of Biobased Copolycarbonates from 1,4:3,6-Dianhydrohexitols. J. Appl. Polym. Sci. 2011, 121, 1450–1463. [Google Scholar] [CrossRef]
- Naves, A.F.; Fernandes, H.T.C.; Immich, A.P.S.; Catalani, L.H. Enzymatic syntheses of unsaturated polyesters based on isosorbide and isomannide. J. Polym. Sci. Part A-Polym. Chem. 2013, 51, 3881–3891. [Google Scholar] [CrossRef]
- Fletcher, H.G.; Goepp, R.M. HEXITOL ANHYDRIDES-1,4,3,6-DIANHYDRO-L-IDITOL AND THE STRUCTURES OF ISOMANNIDE AND ISOSORBIDE. J. Am. Chem. Soc. 1946, 68, 939–941. [Google Scholar] [CrossRef]
- Hockett, R.C.; Fletcher, H.G.; Sheffield, E.L.; Goepp, R.M. HEXITOL ANHYDRIDES—THE STRUCTURE OF ISOSORBIDE, A CRYSTALLINE DIANHYDROSORBITOL. J. Am. Chem. Soc. 1946, 68, 927–930. [Google Scholar] [CrossRef]
- Rose, M.; Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications—Quo vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef]
- Fleche, G.; Fuertes, P.; Tamion, R.; Wyart, H. Method for Purifying a Composition Containing at Least an Internal Dehydration Product for a Hydrogenated Sugar. US7122661B2, 30 May 2001. [Google Scholar]
- Keskivali, J.; Rautiainen, S.; Heikkila, M.; Myllymaki, T.T.T.; Karjalainen, J.P.; Lagerblom, K.; Kemell, M.; Vehkamaki, M.; Meinander, K.; Repo, T. Isosorbide synthesis from cellulose with an efficient and recyclable ruthenium catalyst. Green Chem. 2017, 19, 4563–4570. [Google Scholar] [CrossRef]
- Saska, J.; Dutta, S.; Kindler, A.; Zuend, S.J.; Mascal, M. Efficient and scalable production of isoidide from isosorbide. ACS Sustain. Chem. Eng. 2021, 9, 11565–11570. [Google Scholar] [CrossRef]
- Aricò, F. Isosorbide as biobased platform chemical: Recent advances. Curr. Opin. Green Sustain. Chem. 2020, 21, 82–88. [Google Scholar] [CrossRef]
- Saxon, D.J.; Luke, A.M.; Sajjad, H.; Tolman, W.B.; Reineke, T.M. Next-generation polymers: Isosorbide as a renewable alternative. Prog. Polym. Sci. 2020, 101, 101196. [Google Scholar] [CrossRef]
- Chasseaud, L.F.; Down, W.H.; Grundy, R.K. Concentrations of vasodilator isosorbide dinitrate and its metabolites in blood of human subjects. Eur. J. Clin. Pharmacol. 1975, 8, 157–160. [Google Scholar] [CrossRef]
- Arslan, K.; Erenoglu, B.; Dogru, O.; Kokcam, S.; Turan, E.; Atay, A. Effect of Chronic Anal Fissure Components on Isosorbide Dinitrate Treatment. World J. Surg. 2012, 36, 2225–2229. [Google Scholar] [CrossRef]
- Vogler, M.; Koert, U.; Harms, K.; Dorsch, D.; Gleitz, J.; Raddatz, P. Dianhydrohexitole-based benzamidines: An efficient synthesis of new factor Xa inhibitors. Synth.-Stuttg. 2004, 2004, 1211–1228. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, G.D.; Tang, L.Q.; Liu, Z.P. Design and synthesis of highly potent HIV-1 protease inhibitors with novel isosorbide-derived P2 ligands. Bioorganic Med. Chem. Lett. 2014, 24, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- Carolan, C.G.; Dillon, G.P.; Khan, D.; Ryder, S.A.; Gaynor, J.M.; Reidy, S.; Marquez, J.F.; Jones, M.; Holland, V.; Gilmer, J.F. Isosorbide-2-benzyl Carbamate-5-salicylate, A Peripheral Anionic Site Binding Subnanomolar Selective Butyrylcholinesterase Inhibitor. J. Med. Chem. 2010, 53, 1190–1199. [Google Scholar] [CrossRef]
- Jones, M.; Inkielewicz, I.; Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Radomski, M.W.; Lally, M.N.; Moriarty, L.M.; Gaynor, J.; Carolan, C.G.; et al. Isosorbide-Based Aspirin Prodrugs: Integration of Nitric Oxide Releasing Groups. J. Med. Chem. 2009, 52, 6588–6598. [Google Scholar] [CrossRef] [PubMed]
- Zia, H.; Ma, J.K.H.; Odonnell, J.P.; Luzzi, L.A. Cosolvency of dimethyl isosorbide for steroid solubility. Pharm. Res. 1991, 8, 502–504. [Google Scholar] [CrossRef]
- Caouthar, A.A.; Loupy, A.; Bortolussi, M.; Blais, J.C.; Dubreucq, L.; Meddour, A. Synthesis and characterization of new polyamides based on diphenylaminoisosorbide. J. Polym. Sci. Part A-Polym. Chem. 2005, 43, 6480–6491. [Google Scholar] [CrossRef]
- Caouthar, A.; Roger, P.; Tessier, M.; Chatti, S.; Blais, J.C.; Bortolussi, M. Synthesis and characterization of new polyamides derived from di(4-cyanophenyl)isosorbide. Eur. Polym. J. 2007, 43, 220–230. [Google Scholar] [CrossRef]
- Chatti, S.; Schwarz, G.; Kricheldorf, H.R. Cyclic and noncyclic polycarbonates of isosorbide (1,4:3,6-dianhydro-D-glucitol). Macromolecules 2006, 39, 9064–9070. [Google Scholar] [CrossRef]
- Besset, C.; Pascault, J.P.; Fleury, E.; Drockenmuller, E.; Bernard, J. Structure-Properties Relationship of Biosourced Stereocontrolled Polytriazoles from Click Chemistry Step Growth Polymerization of Diazide and Dialkyne Dianhydrohexitols. Biomacromolecules 2010, 11, 2797–2803. [Google Scholar] [CrossRef]
- Ji, X.D.; Wang, Z.K.; Yan, J.L.; Wang, Z. Partially bio-based polyimides from isohexide-derived diamines. Polymer 2015, 74, 38–45. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Hillmyer, M.A.; Reineke, T.M. Acrylic Triblock Copolymers Incorporating Isosorbide for Pressure Sensitive Adhesives. Acs Sustain. Chem. Eng. 2016, 4, 3379–3387. [Google Scholar] [CrossRef]
- Sadler, J.M.; Nguyen, A.P.T.; Toulan, F.R.; Szabo, J.P.; Palmese, G.R.; Scheck, C.; Lutgen, S.; La Scala, J.J. Isosorbide-methacrylate as a bio-based low viscosity resin for high performance thermosetting applications. J. Mater. Chem. A 2013, 1, 12579–12586. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Garcia, F.G.; Soares, B.G.; Pita, V.J.R.R.; Sánchez, R.; Rieumont, J. Mechanical properties of epoxy networks based on DGEBA and aliphatic amines. J. Appl. Polym. Sci. 2007, 106, 2047–2055. [Google Scholar] [CrossRef]
- Resnik, D.B.; Elliott, K.C. Bisphenol A and risk management ethics. Bioethics 2015, 29, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.S.; Liao, C.Y.; Kannan, K.; Harris, C.; Dolinoy, D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 2015, 124, 54–60. [Google Scholar] [CrossRef]
- Ruan, T.; Liang, D.; Song, S.J.; Song, M.Y.; Wang, H.L.; Jiang, G.B. Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 2015, 124, 150–155. [Google Scholar] [CrossRef]
- Niu, Y.M.; Zhang, J.; Duan, H.J.; Wu, Y.N.; Shao, B. Bisphenol A and nonylphenol in foodstuffs: Chinese dietary exposure from the 2007 total diet study and infant health risk from formulas. Food Chem. 2015, 167, 320–325. [Google Scholar] [CrossRef]
- Lukaszczyk, J.; Janicki, B.; Kaczmarek, M. Synthesis and properties of isosorbide based epoxy resin. Eur. Polym. J. 2011, 47, 1601–1606. [Google Scholar] [CrossRef]
- Chrysanthos, M.; Galy, J.; Pascault, J.P. Preparation and properties of bio-based epoxy networks derived from isosorbide diglycidyl ether. Polymer 2011, 52, 3611–3620. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Varkey, E.C.; Sreekumar, K. Isosorbide based chiral polyurethanes: Optical and thermal studies. J. Mater. Sci. 2010, 45, 1912–1920. [Google Scholar] [CrossRef]
- Zenner, M.D.; Xia, Y.; Chen, J.S.; Kessler, M.R. Polyurethanes from Isosorbide-Based Diisocyanates. Chemsuschem 2013, 6, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Javni, I.; Bilic, O.; Bilic, N.; Petrovic, Z.S.; Eastwood, E.A.; Zhang, F.; Ilavsky, J. Thermoplastic polyurethanes with isosorbide chain extender. J. Appl. Polym. Sci. 2015, 132, 42830. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kang, M.S.; Knowles, J.C.; Gong, M.S. Synthesis of bio-based thermoplastic polyurethane elastomers containing isosorbide and polycarbonate diol and their biocompatible properties. J. Biomater. Appl. 2015, 30, 327–337. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, M.S.; Knowles, J.C.; Gong, M.S. Synthesis of highly elastic biocompatible polyurethanes based on bio-based isosorbide and poly(tetramethylene glycol) and their properties. J. Biomater. Appl. 2014, 29, 454–464. [Google Scholar] [CrossRef]
- Li, C.; Dai, J.Y.; Liu, X.Q.; Jiang, Y.H.; Ma, S.Q.; Zhu, J. Green Synthesis of a Bio-Based Epoxy Curing Agent from Isosorbide in Aqueous Condition and Shape Memory Properties Investigation of the Cured Resin. Macromol. Chem. Phys. 2016, 217, 1439–1447. [Google Scholar] [CrossRef]
- Petrovic, Z.S.; Ionescu, M.; Milic, J.; Eastwood, E. Curing and properties of isosorbide-based epoxy resins. Abstr. Pap. Am. Chem. Soc. 2013, 245, 1155. [Google Scholar]
- Hong, J.; Radojcic, D.; Ionescu, M.; Petrovic, Z.S.; Eastwood, E. Advanced materials from corn: Isosorbide-based epoxy resins. Polym. Chem. 2014, 5, 5360–5368. [Google Scholar] [CrossRef]
- Ristic, I.S.; Radusin, T.; Pilic, B.; Cakic, S.; Budinski-Simendic, J. The Influence of Isosorbide on Thermal Properties of Poly(L-lactide) Synthesized by Different Methods. Polym. Eng. Sci. 2013, 53, 1374–1382. [Google Scholar] [CrossRef]
- Yoon, W.J.; Oh, K.S.; Koo, J.M.; Kim, J.R.; Lee, K.J.; Im, S.S. Advanced Polymerization and Properties of Biobased High T-g polyester of Isosorbide and 1,4-Cyclohexanedicarboxylic Acid through in Situ Acetylation. Macromolecules 2013, 46, 2930–2940. [Google Scholar] [CrossRef]
- Kang, H.L.; Li, X.; Xue, J.J.; Zhang, L.Q.; Liu, L.; Xu, R.W.; Guo, B.C. Preparation and characterization of high strength and noncytotoxic bioelastomers containing isosorbide. Rsc Adv. 2014, 4, 19462–19471. [Google Scholar] [CrossRef]
- Storbeck, R.; Rehahn, M.; Ballauff, M. SYNTHESIS AND PROPERTIES OF HIGH-MOLECULAR-WEIGHT POLYESTERS BASED ON 1,4-3,6-DIANHYDROHEXITOLS AND TEREPHTHALIC ACID. Makromol. Chem.-Macromol. Chem. Phys. 1993, 194, 53–64. [Google Scholar] [CrossRef]
- Majdoub, M.; Loupy, A.; Fleche, G. New polyethers and polyesters from isosorbide—Synthesis and characterization. Eur. Polym. J. 1994, 30, 1431–1437. [Google Scholar] [CrossRef]
- Rajput, B.S.; Gaikwad, S.R.; Menon, S.K.; Chikkali, S.H. Sustainable polyacetals from isohexides. Green Chem. 2014, 16, 3810–3818. [Google Scholar] [CrossRef]
- Hammami, N.; Jarroux, N.; Robitzer, M.; Majdoub, M.; Habas, J.P. Optimized synthesis according to one-step process of a biobased thermoplastic polyacetal derived from isosorbide. Polymers 2016, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Vannini, M.; Marchese, P.; Minesso, A.; Cavalieri, R.; Colonna, M.; Celli, A. Sustainable polyesters for powder coating applications from recycled PET, isosorbide and succinic acid. Green Chem. 2014, 16, 1807–1815. [Google Scholar] [CrossRef]
- Noordover, B.A.J.; van Staalduinen, V.G.; Duchateau, R.; Koning, C.E.; van Benthem; Mak, M.; Heise, A.; Frissen, A.E.; van Haveren, J. Co- and Terpolyesters Based on Isosorbide and Succinic Acid for Coating Applications: Synthesis and Characterization. Biomacromolecules 2006, 7, 3406–3416. [Google Scholar] [CrossRef]
- Thiem, J.; Luders, H. Synthesis of polyterephthalates derived from dianhydrohexitols. Polym. Bull. 1984, 11, 365–369. [Google Scholar] [CrossRef]
- Noordover, B.A.J.; Sablong, R.J.; Duchateau, R.; van Benthem, R.A.T.M.; Ming, W.; Koning, C.E.; van Haveren, J. Process for the Production of a Dianhydrohexitol Based Polyester. WO2008031592, 1 January 2008. [Google Scholar]
- Charbonneau, L.F.; Dalickas, G. Processes for Making Low Color Poly(ethylene-co-isosorbide) Terephtalate Polymers. WO 2006/032022 A1, 23 March 2006. [Google Scholar]
- Adelman, J.D.; Charbonneau, L.F.; Ung, S. Process for Making Poly(ethylene-co-isosorbide) Terephtalate Polymer. US 6656577 B1, 2 December 2003. [Google Scholar]
- Li, D.; Li, Y.B.; Zhang, Y.; Xu, Y.S.; Zhang, X.M.; Hakkarainen, M. Designing Biobased Poly(ethylene-co-isosorbide terephthalate) Copolyesters with Tunable Properties and Degradability. Biomacromolecules 2025, 26, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Carbohydrate-Based Building Blocks and Step-growth Polymers Synthesis, Characterization and Structure-Properties Relations. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2012. [Google Scholar]
- Yan, S.D.; Wu, G.Z. Thermo-induced chain scission and oxidation of isosorbide-based polycarbonates: Degradation mechanism and stabilization strategies. Polym. Degrad. Stab. 2022, 202, 110028. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules, 3rd ed.; Springer: London, UK, 2013. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; p. 464. [Google Scholar]
- Chatti, S.; Bortolussi, M.; Bogdal, D.; Blais, J.C.; Loupy, A. Microwave-assisted polycondensation of aliphatic diols of isosorbide with aliphatic disulphonylesters via phase-transfer catalysis. Eur. Polym. J. 2004, 40, 561–577. [Google Scholar] [CrossRef]
- Chatti, S.; Bortolussi, M.; Bogdal, D.; Blais, J.C.; Loupy, A. Synthesis and properties of new poly(ether-esters containing aliphatic diol based on isosorbide. Effects of the microwave-assisted polycondensation. Eur. Polym. J. 2006, 42, 410–424. [Google Scholar] [CrossRef]
- Chatti, S.; Kricheldorf, H.R. Copolyesters of epsilon-caprolactone, isosorbide and suberic acid by ring-opening copolymerization. J. Macromol. Sci. Part A-Pure Appl. Chem. 2006, 43, 967–975. [Google Scholar] [CrossRef]
- Chiarelli, S.N.; Rossi, M.T.; Pizzorno, M.T.; Albonico, S.M. NMR Determination of isosorbide dinitrate and beta-adrenergic blocking-agents in tablets. J. Pharm. Sci. 1982, 71, 1178–1180. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Sun, S.J.; Chen, C.P.; Chang, T.C. Polymers of carbonic acid, 24. Photoreactive, nematic or cholesteric polycarbonates derived from hydroquinone-4-hydroxybenzoate 4,4’-dihydroxychalcone and isosorbide. J. Polym. Sci. Part A-Polym. Chem. 1997, 35, 1611–1619. [Google Scholar] [CrossRef]
- Sun, S.J.; Schwarz, G.; Kricheldorf, H.R.; Chang, T.C. New polymers of carbonic acid. XXV. Photoreactive cholesteric polycarbonates derived from 2,5-bis(4‘-hydroxybenzylidene)cyclopentanone and isosorbide. J. Polym. Sci. Part A-Polym. Chem. 1999, 37, 1125–1133. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Zheng, Y.Y.; Xu, Y.; Lu, H.W. Synthesis and characterization of side chain cholesteric liquid crystalline polymers containing isosorbide as a chiral centre. Liq. Cryst. 2005, 32, 357–364. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Sun, S.J.; Sapich, B.; Stumpe, J. Polymers of carbonic acid, 23. Photoreactive cholesteric polycarbonates based on isosorbide, 4,4’-dihydroxychalcone and 4,4’-dihydroxybiphenyl. Macromol. Chem. Phys. 1997, 198, 2197–2210. [Google Scholar] [CrossRef]
- Beghdadi, S.; Miladi, I.A.; Ben Romdhane, H.; Bernard, J.; Drockenmuller, E. RAFT Polymerization of Bio-Based 1-Vinyl-4-dianhydrohexitol-1,2,3-triazole Stereoisomers Obtained via Click Chemistry. Biomacromolecules 2012, 13, 4138–4145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.P.; Ma, D.Z. Double cold crystallization peaks of poly(ethylene terephthalate)-1. Samples isothermally crystallized at low temperature. Eur. Polym. J. 1997, 33, 1817–1818. [Google Scholar] [CrossRef]
- Ling, X.Y.; Spruiell, J.E. Analysis of the complex thermal behavior of poly(L-lactic acid) film. I. Samples crystallized from the glassy state. J. Polym. Sci. Part B-Polym. Phys. 2006, 44, 3200–3214. [Google Scholar] [CrossRef]
- Liu, T.X.; Mo, Z.S.; Wang, S.G.; Zhang, H.F. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Abiodun, S.; Krishnamoorti, R.; Bhowmick, A.K. Thermal degradation of polytetrafluoroethylene, modified polytetrafluoroethylene, and their nanocomposites with boron nitride nanobarbs: Lifetime predictions and kinetic analysis. J. Appl. Polym. Sci. 2025, 142, e56452. [Google Scholar] [CrossRef]
- Qian, J.; Fu, C.; Wu, X.Y.; Ran, X.H.; Nie, W. Promotion of poly(vinylidene fluoride) on thermal stability and rheological property of ethylene-tetrafluoroethylene copolymer. e-Polymers 2018, 18, 541–549. [Google Scholar] [CrossRef]
- Hammami, N.; Majdoub, M.; Habas, J.-P. Structure-properties relationships in isosorbide-based polyacetals: Influence of linear or cyclic architecture on polymer physicochemical properties. Eur. Polym. J. 2017, 93, 795–804. [Google Scholar] [CrossRef]
- Stampfer, L.; Bouilhac, C.; Mérian, T.; Chabert, F.; Djilali, T.; Nassiet, V.; Habas, J.P. Investigation of the Reaction between a Homemade PEEK Oligomer and an Epoxy Prepolymer: Optimisation of Critical Parameters Using Physico-Chemical Methods. Polymers 2024, 16, 764. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Calleja, G.; Jourdan, A.; Ameduri, B.; Habas, J.P. Where is the glass transition temperature of poly(tetrafluoroethylene)? A new approach by dynamic rheometry and mechanical tests. Eur. Polym. J. 2013, 49, 2214–2222. [Google Scholar] [CrossRef]
- Plante, A.; Palato, S.; Lebel, O.; Soldera, A. Functionalization of molecular glasses: Effect on the glass transition temperature. J. Mater. Chem. C 2013, 1, 1037–1042. [Google Scholar] [CrossRef]
- Johari, G.P. INTRINSIC MOBILITY OF MOLECULAR GLASSES. J. Chem. Phys. 1973, 58, 1766–1770. [Google Scholar] [CrossRef]
- Correia, N.T.; Alvarez, C.; Ramos, J.J.M.; Descamps, M. Molecular motions in molecular glasses as studied by thermally stimulated depolarisation currents (TSDC). Chem. Phys. 2000, 252, 151–163. [Google Scholar] [CrossRef]
- Laventure, A.; De Grandpré, G.; Soldera, A.; Lebel, O.; Pellerin, C. Unraveling the interplay between hydrogen bonding and rotational energy barrier to fine-tune the properties of triazine molecular glasses. Phys. Chem. Chem. Phys. 2016, 18, 1681–1692. [Google Scholar] [CrossRef]

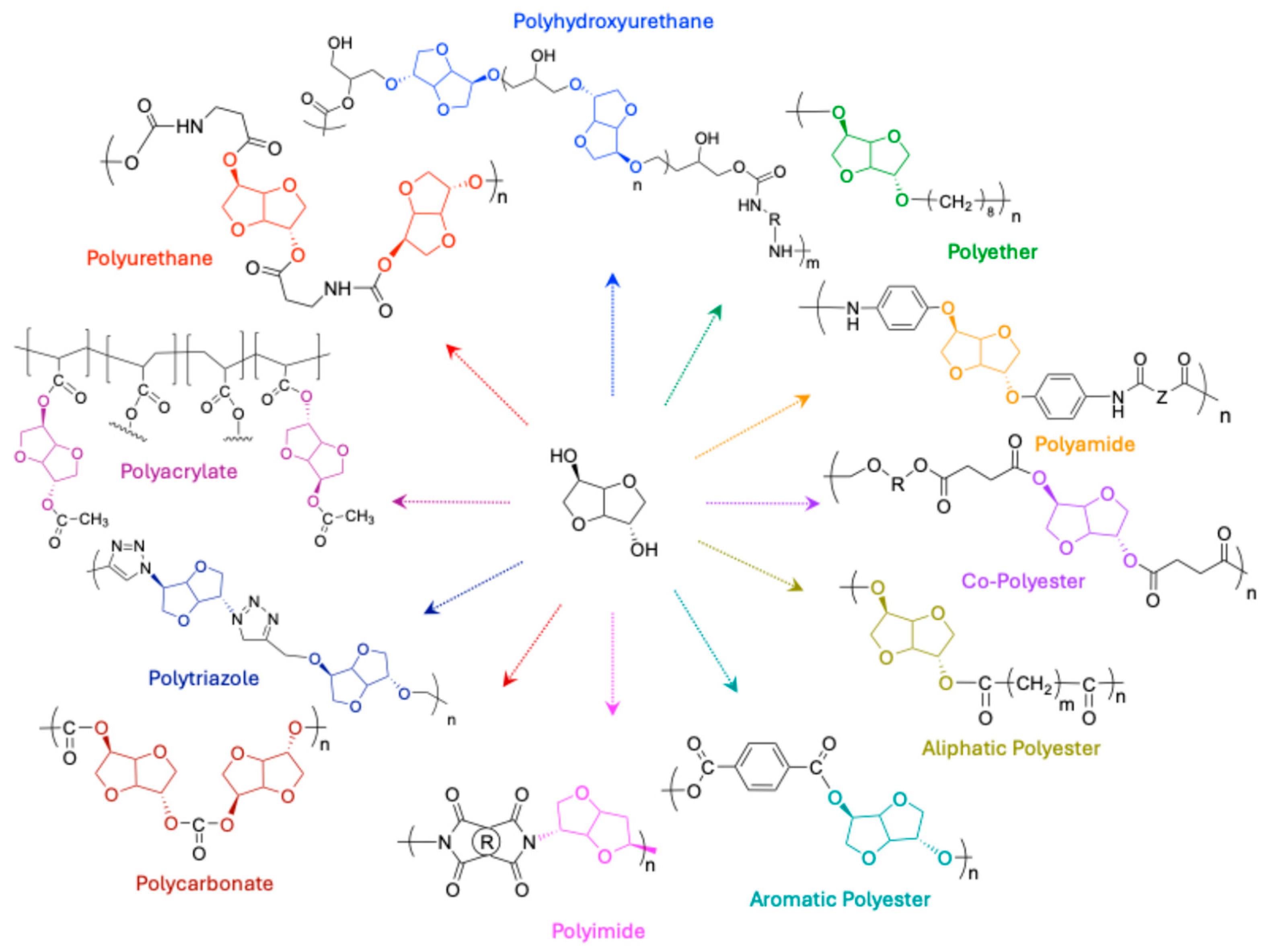
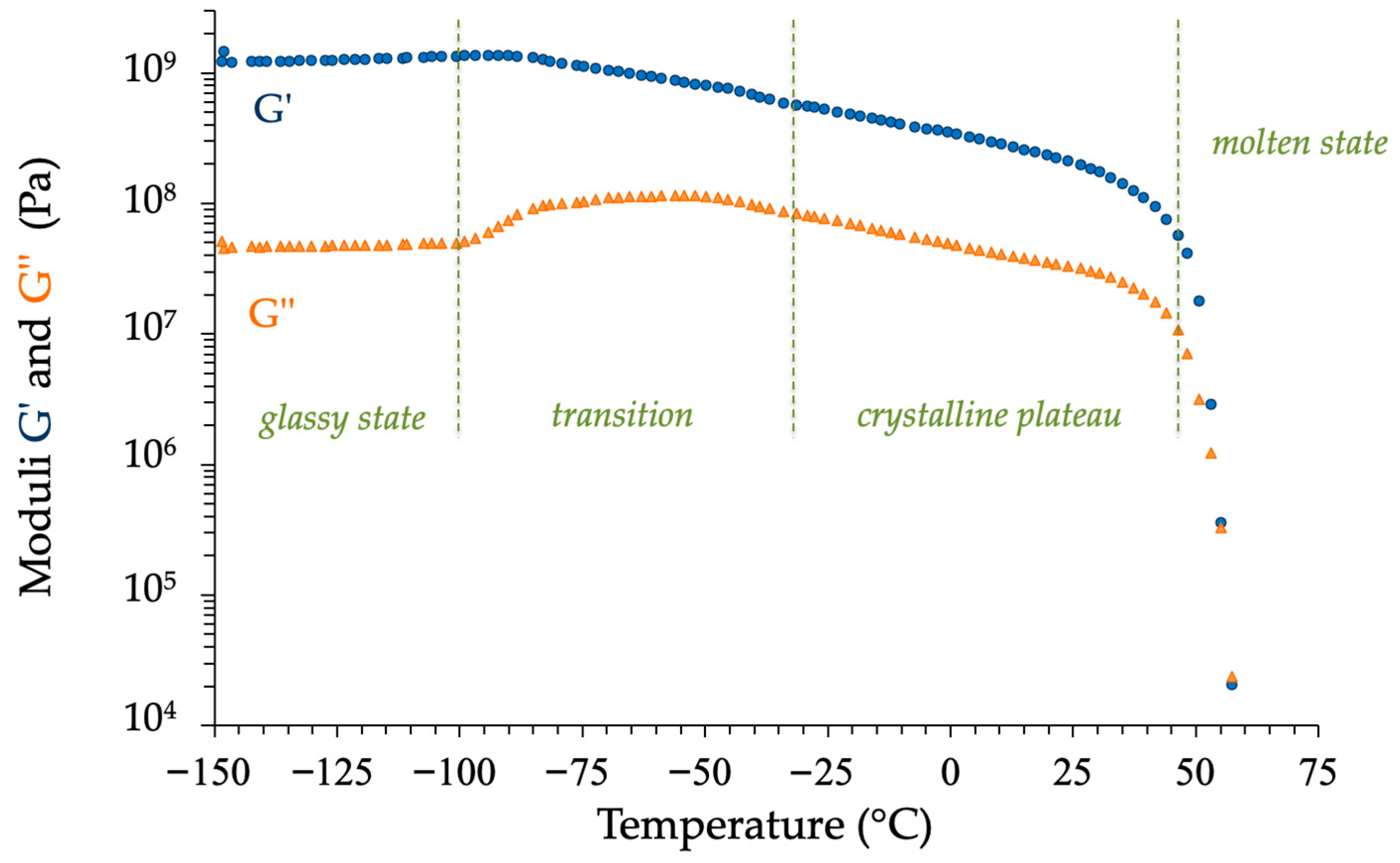
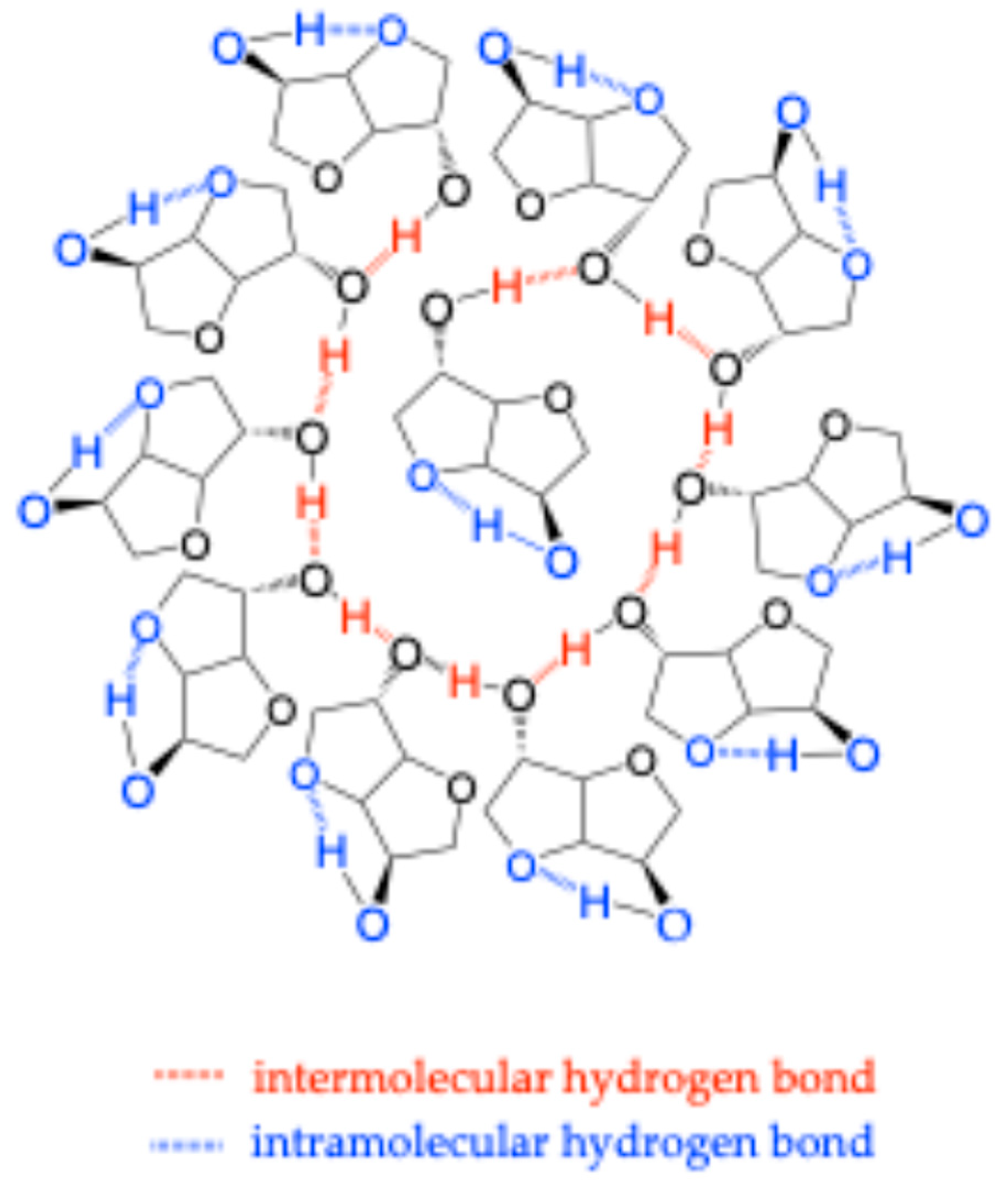

| N° | Polymer | Synthesis Conditions | T (°C) | Time (h) | Mn (g/mol) | Color | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | Poly(isosorbide-terephthalate) | in bulk | 180 | 0.17 | 3000 | colorless | [56] |
| 2 | Poly(ethylene-co-isosorbide) terephthalate | in bulk | 180–265 | 4 | 4000 | yellow | [60,61] |
| 3 | Poly(isosorbide-succinate) | in bulk | 180 | 4 | 2000 | yellow | [55,57] |
| 4 | Polycarbonate | in bulk with diphenyl carbonate | 160–245 | 4 | 3200 | yellow | [6] |
| 5 | Polycarbonate | in bulk with diphosgene and pyridine | 20 | 24 | 50,000 | colorless | [25] |
| 6 | Polyurethane | in bulk | 70 | 24 | 50,000 | colorless | [39] |
| 7 | Aromatic and linear polyether | solid–liquid two phases mixing | 80 | 4–20 | 300–1000 | brown | [51] |
| 8 | Aliphatic polyacetal | solvent (DMSO) | 90 | 0.08 | 9000 | colorless | [53] |
| Wavenumber (cm−1) | Assignment | Notation | Intensity |
|---|---|---|---|
| 3600–3200 | O–H stretching (H bonded) | ν(OH) | br, s |
| 2970–2940 | CH stretching (aliphatic, CH/CH2) | ν(CH) | m |
| 2945–2905 | Asymmetric CH2 stretching | ν_as(CH2) | m |
| 2870–2840 | Symmetric CH2 stretching | ν_s(CH2) | m |
| 1640–1610 | H–O–H bending (reduced traces of moisture) | δ (H–O–H) | w |
| 1430–1460 | HCH bending | δ(HCH) | m |
| 1410 ± 20 | In place O–H bending | δ(COH) | w–m |
| 1400–1300 | CH2 wagging/twisting | ρ/τ(CH2) | w–m |
| 1200–1140 | C–O–C stretching (asymmetric) | ν_as(C–O–C) | s |
| 1080–1040 | C–O–C stretching (symmetric) | ν_s(C–O–C) | m–s |
| 1070–1020 | C–O stretching (secondary alcohol) | ν(C–O) | m–s |
| 1000–920 | Ring/skeletal C–C–O and C–C modes | ν(skeletal) | w–m |
| 900–650 | Out of plane C–H bends and rocking | γ(C–H) | s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammami, N.; Patry, S.; Soldera, A.; Ameduri, B.; Habas, J.-P. Isosorbide as a Molecular Glass: New Insights into the Physicochemical Behavior of a Biobased Diol. Molecules 2025, 30, 4364. https://doi.org/10.3390/molecules30224364
Hammami N, Patry S, Soldera A, Ameduri B, Habas J-P. Isosorbide as a Molecular Glass: New Insights into the Physicochemical Behavior of a Biobased Diol. Molecules. 2025; 30(22):4364. https://doi.org/10.3390/molecules30224364
Chicago/Turabian StyleHammami, Nadia, Stéphane Patry, Armand Soldera, Bruno Ameduri, and Jean-Pierre Habas. 2025. "Isosorbide as a Molecular Glass: New Insights into the Physicochemical Behavior of a Biobased Diol" Molecules 30, no. 22: 4364. https://doi.org/10.3390/molecules30224364
APA StyleHammami, N., Patry, S., Soldera, A., Ameduri, B., & Habas, J.-P. (2025). Isosorbide as a Molecular Glass: New Insights into the Physicochemical Behavior of a Biobased Diol. Molecules, 30(22), 4364. https://doi.org/10.3390/molecules30224364








