The Effects of New Zealand Grown Ginseng Fractions on Cytokine Production from Human Monocytic THP-1 Cells
Abstract
:1. Introduction
2. Results
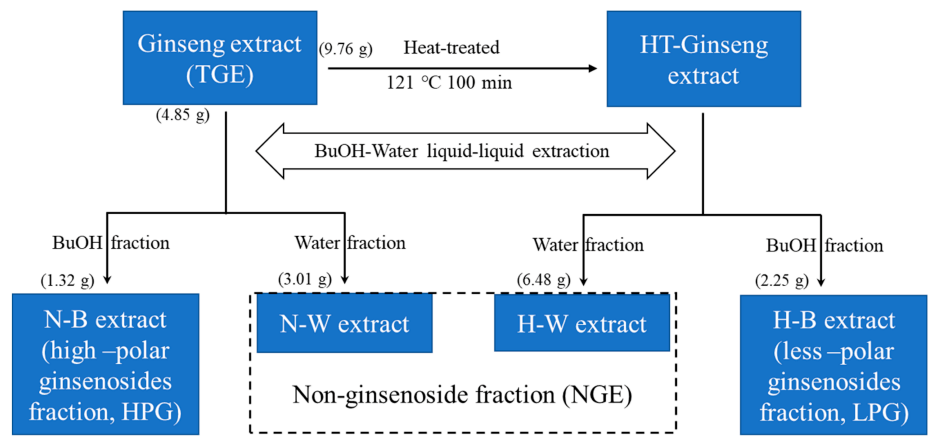
2.1. Ginsenoside Profiles of Ginseng Extract Fractions
2.2. Quantification of Ginsenosides in the Ginseng Fraction Extracts
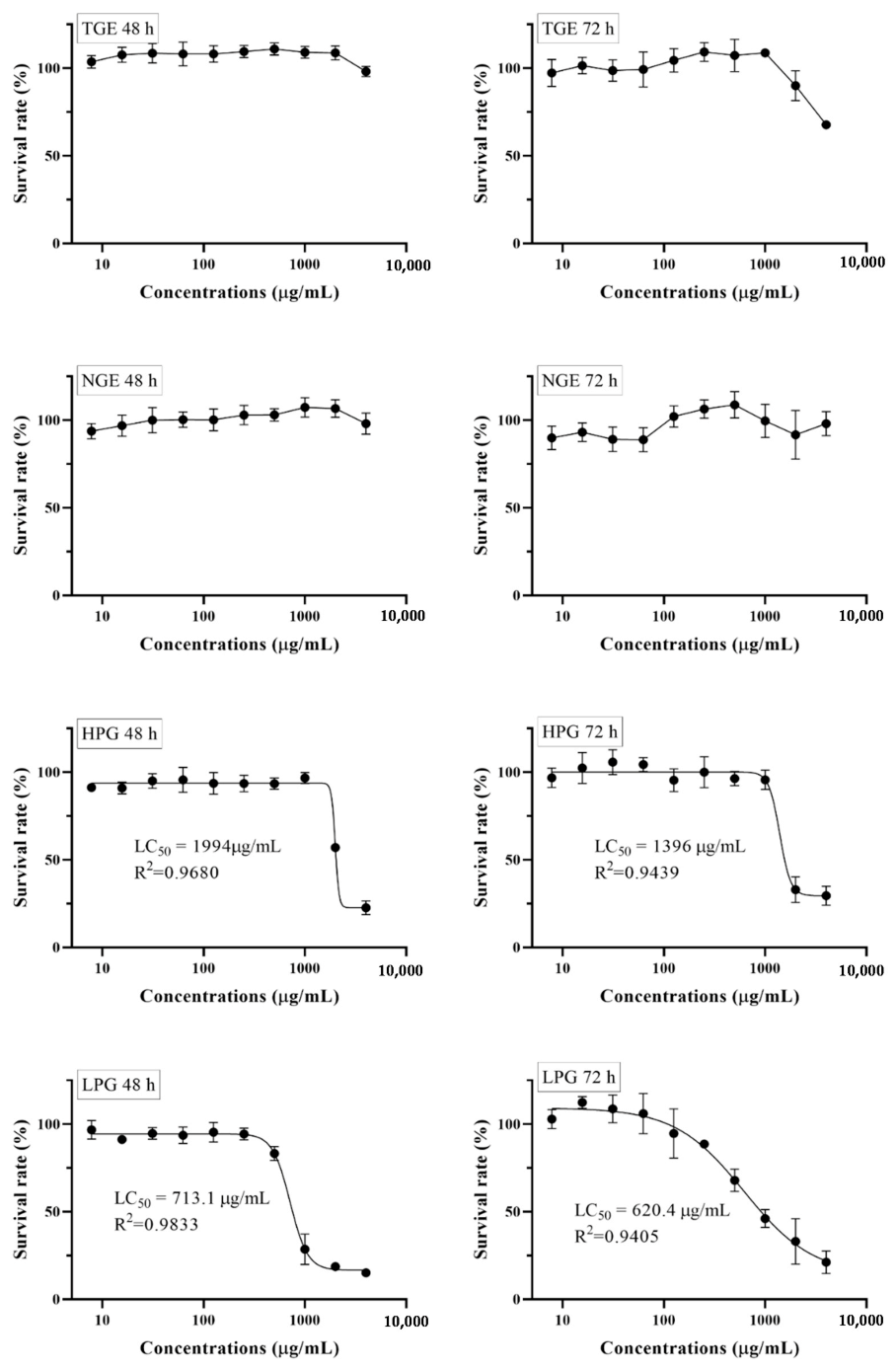
2.3. The Effect of Different Ginseng Fraction Extracts on Cell Viability
2.4. The Effect of Different Ginseng Fraction Extracts on Cell Viability
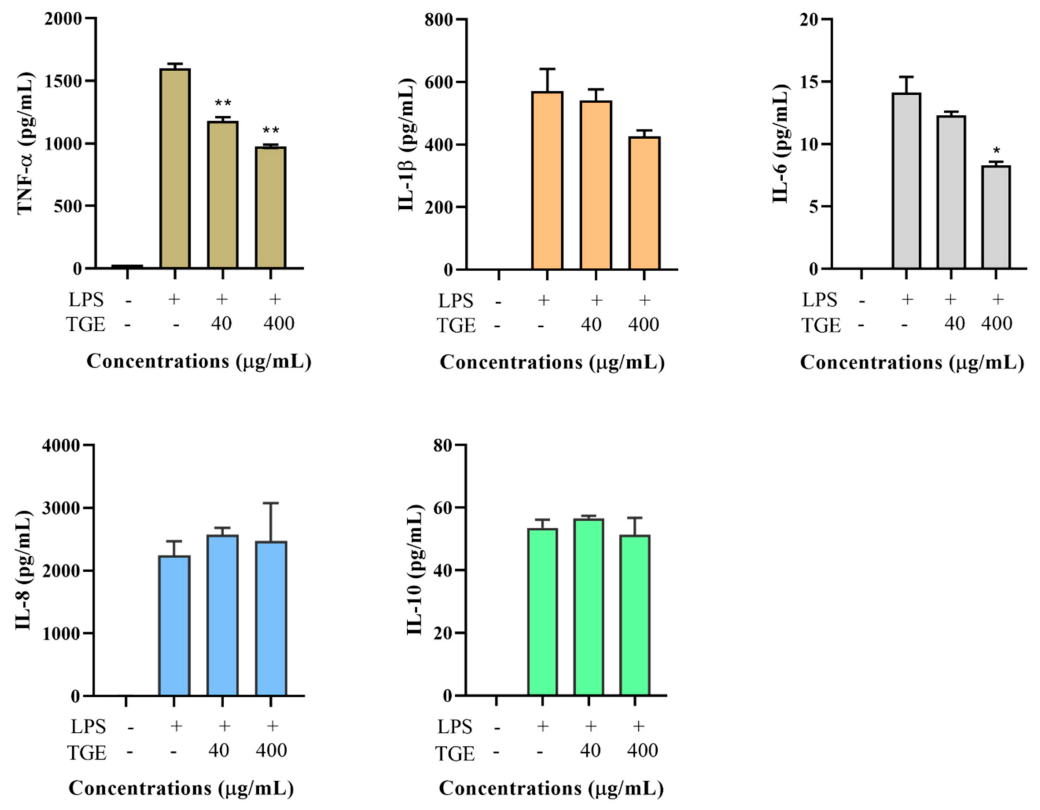
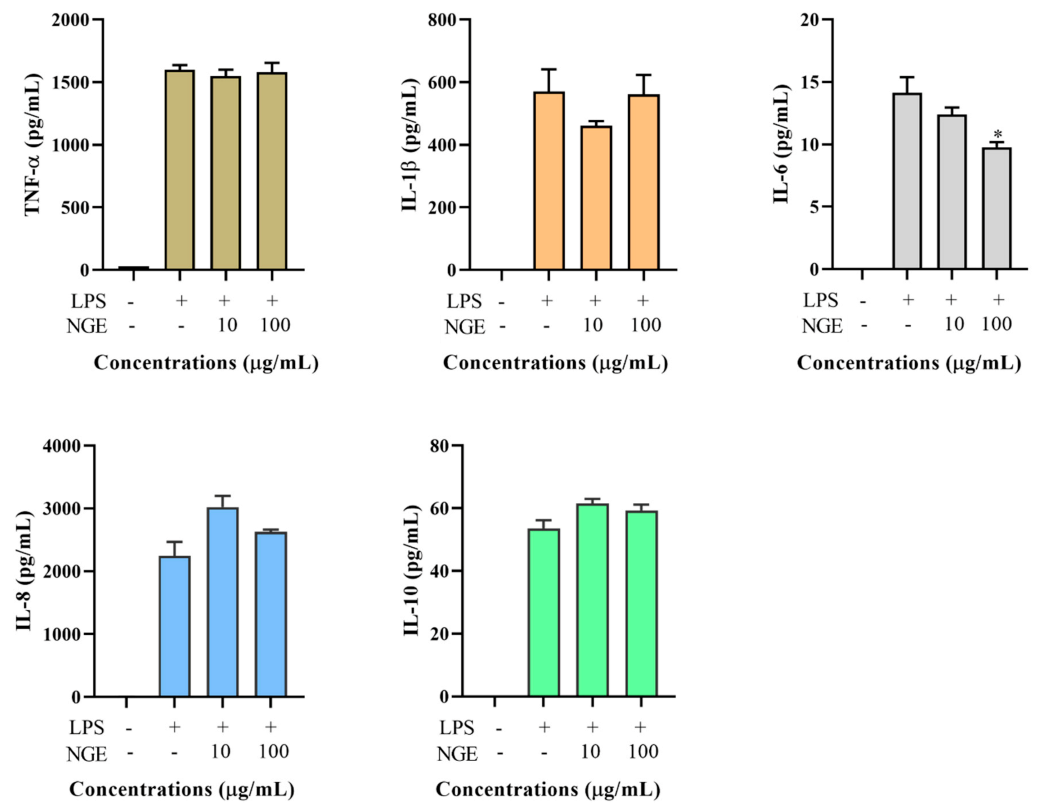
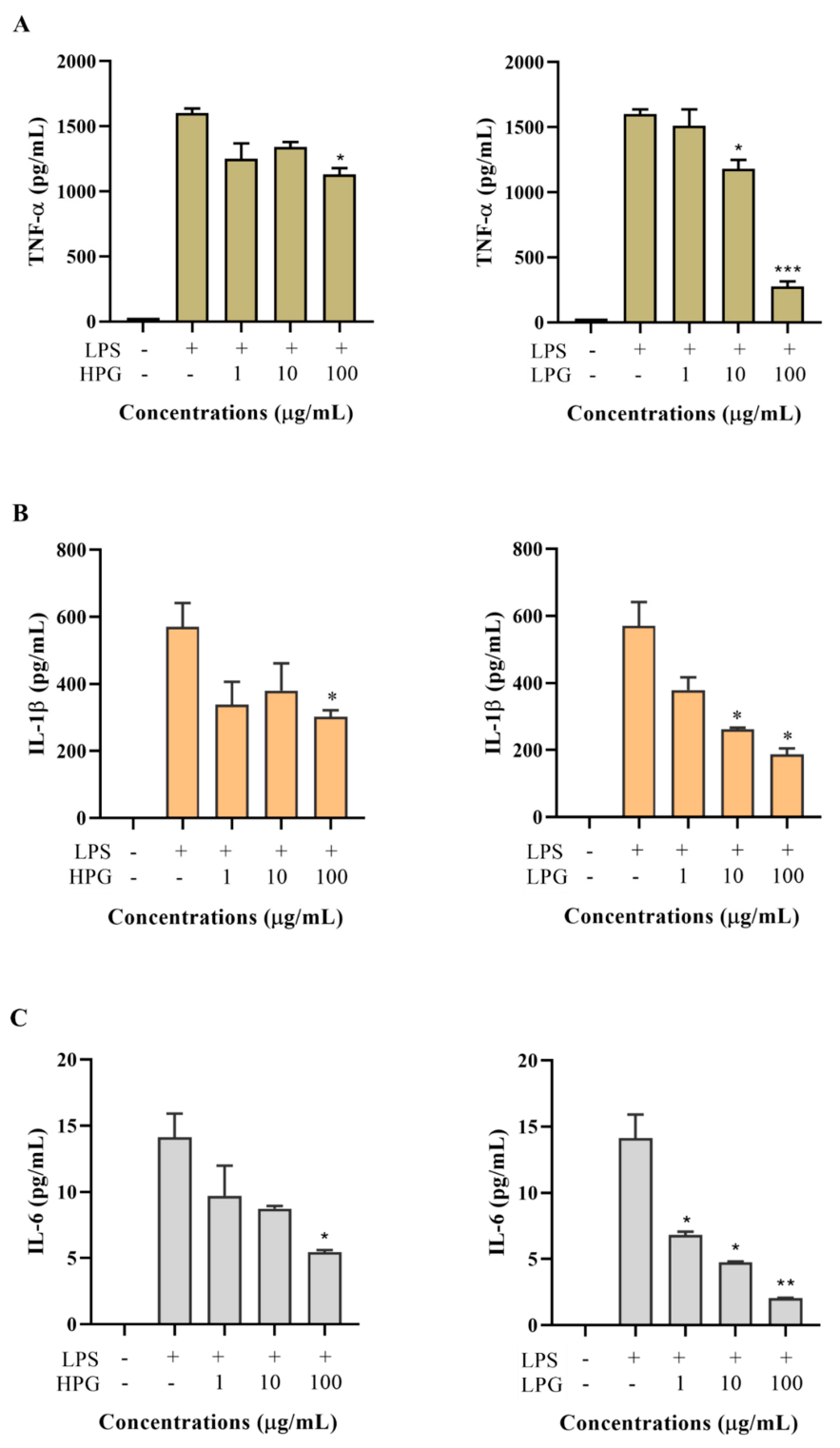
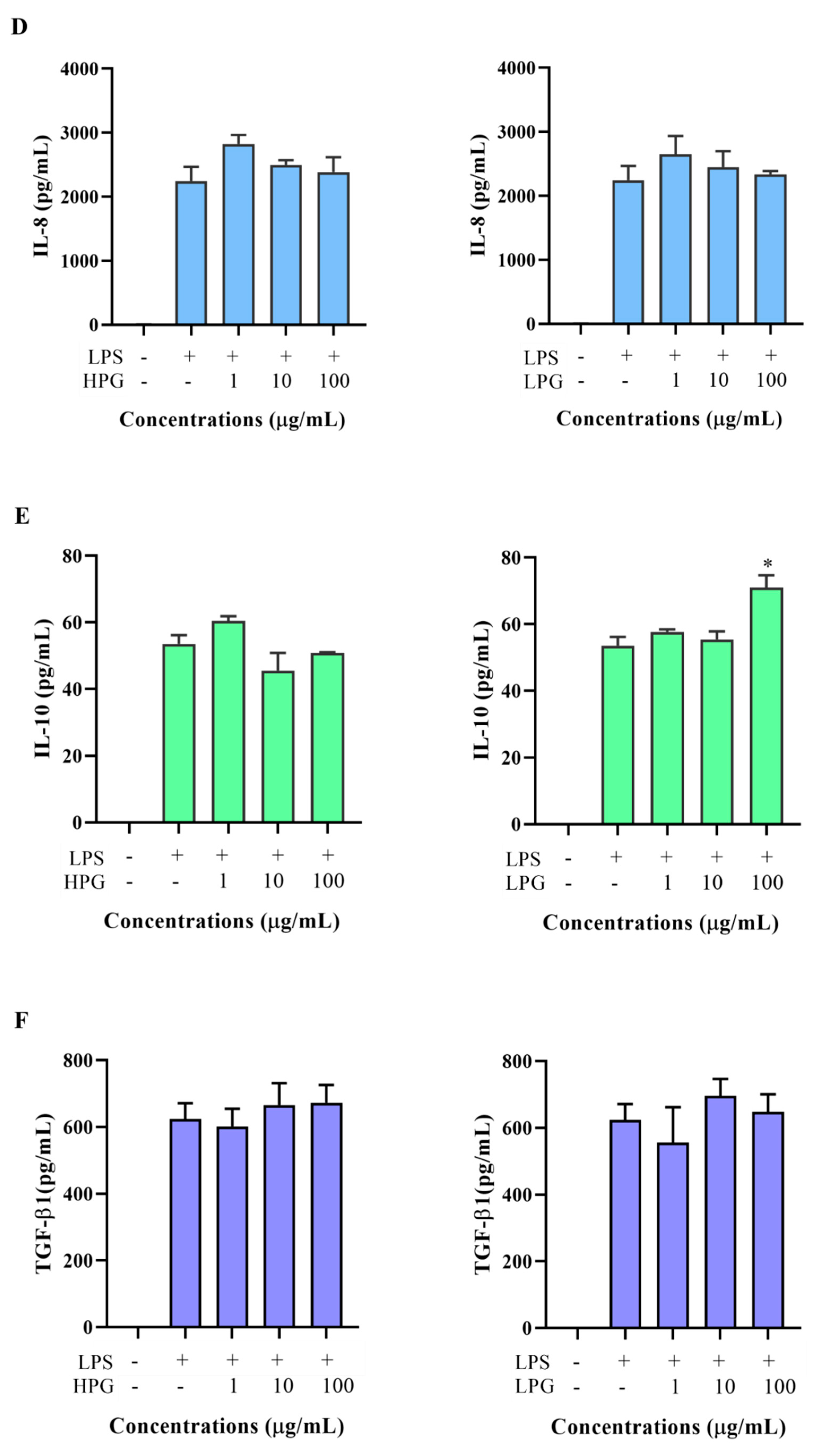
2.5. Effects of Non-Ginsenoside Fraction Extract (NGE) on Cytokine Production
2.6. Effects of Ginsenoside Fraction Extracts on Cytokine Production
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Ginseng Materials
4.3. Cells
4.4. Ginseng Fractions Preparation
4.5. HPLC Analysis
4.6. THP-1 Cell Culture and Treatments
4.7. Cell Viability
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lee, D.C.; Lau, A.S. Effects of Panax ginseng on tumor necrosis factor-alpha-mediated inflammation: A mini-review. Molecules 2011, 16, 2802–2816. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouda, W.; Mageed, L.; Abd El Dayem, S.M.; Ashour, E.; Afify, M. Evaluation of pro-inflammatory and anti-inflammatory cytokines in type 1 diabetes mellitus. Bull. Nat. Res. Cent. 2018, 42, 14. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and anti-inflammaging: The role of cytokines in extreme longevity. Arch Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [Green Version]
- Esser, N.; Paquot, N.; Scheen, A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 2015, 24, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Modi, D.R. Inflammation and diabetes. Interdiscip. J. Microinflam. 2014, 1, 110. [Google Scholar]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 39–50. [Google Scholar] [CrossRef]
- Lee, E.E.; Hong, S.; Martin, A.S.; Eyler, L.T.; Jeste, D.V. Inflammation in schizophrenia: Cytokine levels and their relationships to demographic and clinical variables. Am. J. Geriatr. Psychiatry 2017, 25, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishtar, E.; Jovanovski, E.; Jenkins, A.; Vuksan, V. Effects of Korean white ginseng (Panax Ginseng C.A. Meyer) on vascular and glycemic health in type 2 diabetes: Results of a randomized, double blind, placebo-controlled, multiple-crossover, acute dose escalation trial. Clin. Nutr. Res. 2014, 3, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Balan, P.; Popovich, D.G. Chapter 6-Comparison of the ginsenoside composition of Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) and their transformation pathways. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 161–195. [Google Scholar]
- Sun, J.; Jiao, C.; Ma, Y.; Chen, J.; Wu, W.; Liu, S. Anti-ageing effect of red ginseng revealed by urinary metabonomics using RRLC-Q-TOF-MS. Phytochem. Anal. 2018, 29, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Lee, Y.; Seo, M.K.; Sung, D.J. Anti-fatigue effects of acute red ginseng intake in recovery from repetitive anaerobic exercise. Iran. J. Public Health 2016, 45, 387–389. [Google Scholar]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Amiri, A.; Bordbar, A.K. New generation of drug delivery systems based on ginsenoside Rh2-, Lysine- and Arginine-treated highly porous graphene for improving anticancer activity. Sci. Rep. 2018, 8, 586. [Google Scholar] [CrossRef]
- Saptarshi, P.; Somnath, S. Medicinal plants with anti-diabetic potential. Int. J. Adv. Res. Biol. Sci. 2018, 5, 156–163. [Google Scholar]
- Gao, H.; Kang, N.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 2020, 69, 153197. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Analysis of ginsenoside content (Panax ginseng) from different regions. Molecules 2019, 24, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective effects of Gynostemma pentaphyllum (var. Ginpent) against lipopolysaccharide-induced inflammation and motor alteration in mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kong, G.; Tran, Q.; Kim, C.; Park, J.; Park, J. Relationship between ginsenoside Rg3 and metabolic syndrome. Front. Pharmacol. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Liu, Q.; Wan, J.Y.; Zhao, Y.J.; Guo, R.Z.; Alolga, R.N.; Li, P.; Qi, L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci. Rep. 2015, 5, 8598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Jung, H.G.; Myint, A.M.; Kim, H.; Park, S.H. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007, 104, 91–95. [Google Scholar] [CrossRef]
- Clark, I.A. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.G.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Choi, S.H.; Yu, G.J.; Hong, S.H.; Chung, Y.H.; Kim, C.H.; Yoon, H.M.; Kim, G.Y.; Kim, B.W.; Choi, Y.H. Anti-inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther Med. 2016, 11, 1109–1115. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, L.B.; Qiu, Z.; Chen, X.B.; Liu, Y.; Li, J.; Wang, L.; Wang, Y.P. Ginsenoside Rg1 ameliorates testicular senescence changes in Dgalinduced aging mice via antiinflammatory and antioxidative mechanisms. Mol. Med. Rep. 2018, 17, 6269–6276. [Google Scholar]
- Yu, S.; Zhou, X.; Li, F.; Xu, C.; Zheng, F.; Li, J.; Zhao, H.; Dai, Y.; Liu, S.; Feng, Y. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci. Rep. 2017, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Yang, Y.; Kwak, Y.S.; Song, G.G.; Kim, M.Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J. Ginseng. Res. 2017, 41, 127–133. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.S.; Lee, E.J.; Lee, S.Y.; Kim, D.H.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: Critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015, 63, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Park, J.; Kim, S.H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Ginsenoside Rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol. Ther. 2013, 21, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.Y.; Saba, E.; Irfan, M.; Kim, M.; Chan, J.Y.-L.; Jeon, B.S.; Choi, S.K.; Rhee, M.H. The anti-inflammatory and anti-nociceptive effects of Korean black ginseng. Phytomedicine 2019, 54, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Choi, H.; Lim, H.W.; Shin, D.; Kim, H.; Kwon, B.; Lee, J.E.; Park, E.H.; Lim, C.J. Enhancement of anti-inflammatory and antinociceptive actions of red ginseng extract by fermentation. J. Pharm. Pharmacol. 2012, 64, 756–762. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, S.H.; Choi, B.R.; Choi, D.J.; Lee, Y.G.; Kim, H.G.; Kim, G.S.; Kim, K.; Lee, Y.H.; Baek, N.I.; et al. UPLC-QTOF/MS-based metabolomics applied for the quality evaluation of four processed Panax ginseng products. Molecules 2018, 23, 2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nag, S.A.; Qin, J.J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R. Ginsenosides as anticancer agents: In vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean red ginseng. J. Ginseng. Res. 2020, 44, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, C.Y.; Hwang, J.; Choi, H.S. Non-saponin fraction of red ginseng inhibits monocyte-to-macrophage differentiation and inflammatory responses in vitro. Korean J. Food Sci. Technol. 2019, 51, 70–80. [Google Scholar]
- Park, S.J.; Lee, D.; Kim, D.; Lee, M.; In, G.; Han, S.T.; Kim, S.W.; Lee, M.H.; Kim, O.K.; Lee, J. The non-saponin fraction of Korean Red Ginseng (KGC05P0) decreases glucose uptake and transport in vitro and modulates glucose production via down-regulation of the PI3K/AKT pathway in vivo. J. Ginseng. Res. 2020, 44, 362–372. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, K.H.; Sohn, E.; Park, J.D.; Kim, B.O.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1817–1825. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Changes of ginsenoside composition in the creation of black ginseng leaf. Molecules 2020, 25, 2809. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, Y.C.; Hsiao, H.P.; Kuo, C.H.; Chen, B.H.; Chen, Y.T.; Wang, S.L.; Tsai, M.L.; Hung, C.H. The effects of acarbose on chemokine and cytokine production in human monocytic THP-1 cells. Hormones 2019, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Popovich, D.G.; Hu, C. Characterizing the mechanism for ginsenoside-induced cytotoxicity in cultured leukemia (THP-1) cells. Can. J. Physiol Pharmacol. 2007, 85, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
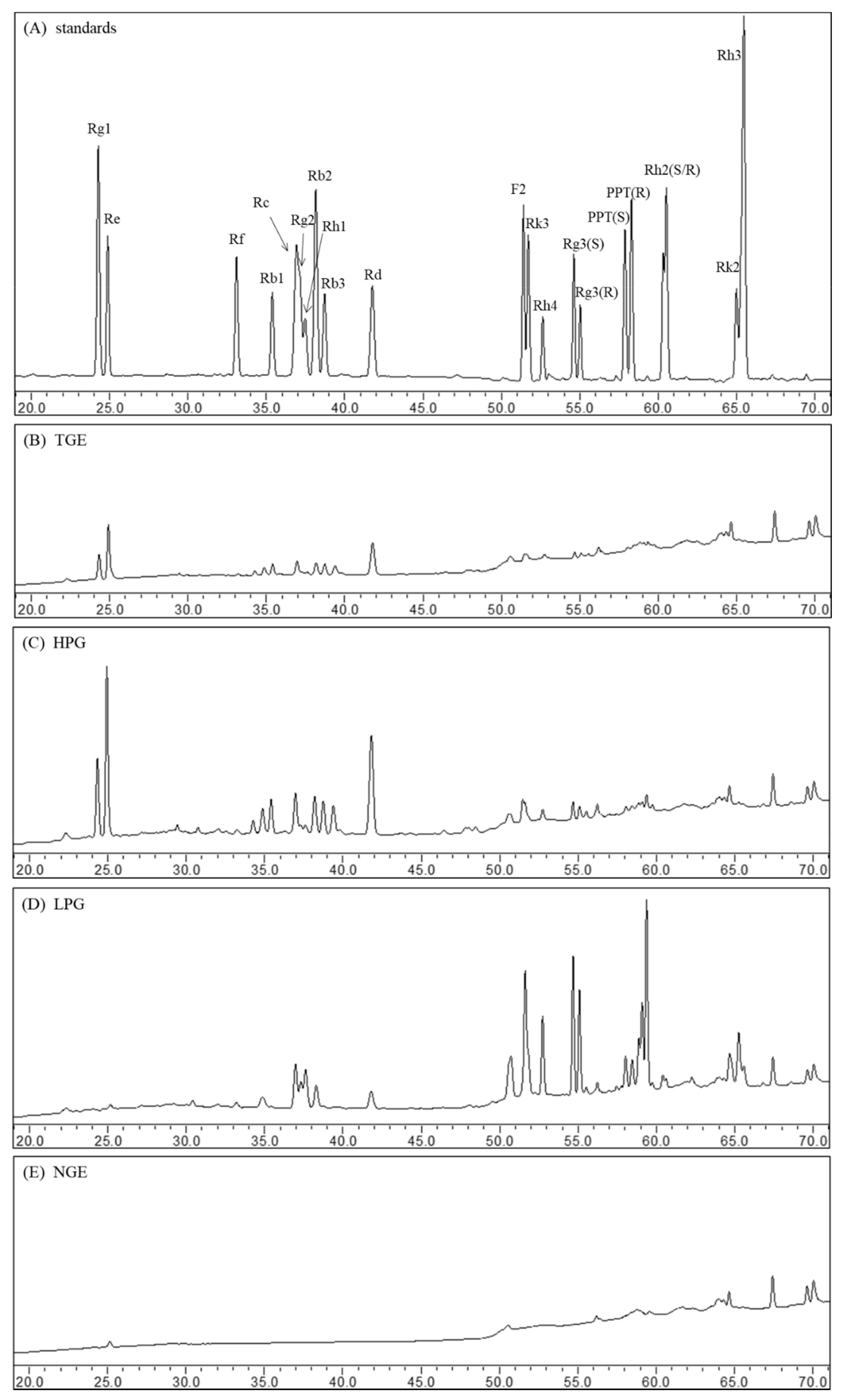
| Ginsenosides | Ginsenoside Content (mg/g) of Ginseng Extracts | ||
|---|---|---|---|
| TGE | HPG | LPG | |
| Rg1 | 12.10 ± 1.5 | 44.99 ± 0.49 | |
| Re | 29.08 ± 2.20 | 97.26 ± 2.08 | |
| Rf | 1.08 ± 0.66 | 5.44 ± 0.66 | |
| Rb1 | 7.46 ± 0.70 | 25.40 ± 1.93 | |
| Rc | 10.63 ± 0.62 | 37.44 ± 0.95 | |
| Rg2 | 26.75 ± 1.17 | ||
| Rh1 | 29.71 ± 0.95 | ||
| Rb2 | 10.12 ± 1.38 | 35.85 ± 0.89 | |
| Rb3 | 8.77 ± 1.33 | 30.00 ± 0.09 | |
| Rd | 26.15 ± 3.56 | 83.62 ± 10.25 | 18.07 ± 1.79 |
| F2 | 74.31 ± 2.15 | ||
| Rk3 | 32.01 ± 2.18 | ||
| Rh4 | 53.03 ± 0.44 | ||
| 20(S)-Rg3 | 51.95 ± 0.10 | ||
| 20(R)-Rg3 | 65.46 ± 0.12 | ||
| 20(S)-PPT | 15.64 ± 0.03 | ||
| 20(S)-Rh2 | 4.43 ± 0.39 | ||
| Rk2 | 43.27 ± 0.25 | ||
| Rh3 | 10.23 ± 0.21 | ||
| Total | 105.39 ± 11.96 | 360.00 ± 3.17 | 424.85 ± 2.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Balan, P.; Popovich, D.G. The Effects of New Zealand Grown Ginseng Fractions on Cytokine Production from Human Monocytic THP-1 Cells. Molecules 2021, 26, 1158. https://doi.org/10.3390/molecules26041158
Chen W, Balan P, Popovich DG. The Effects of New Zealand Grown Ginseng Fractions on Cytokine Production from Human Monocytic THP-1 Cells. Molecules. 2021; 26(4):1158. https://doi.org/10.3390/molecules26041158
Chicago/Turabian StyleChen, Wei, Prabhu Balan, and David G. Popovich. 2021. "The Effects of New Zealand Grown Ginseng Fractions on Cytokine Production from Human Monocytic THP-1 Cells" Molecules 26, no. 4: 1158. https://doi.org/10.3390/molecules26041158






