Coumarins as Powerful Photosensitizers for the Cationic Polymerization of Epoxy-Silicones under Near-UV and Visible Light and Applications for 3D Printing Technology
Abstract
:1. Introduction
2. Results and Discussion
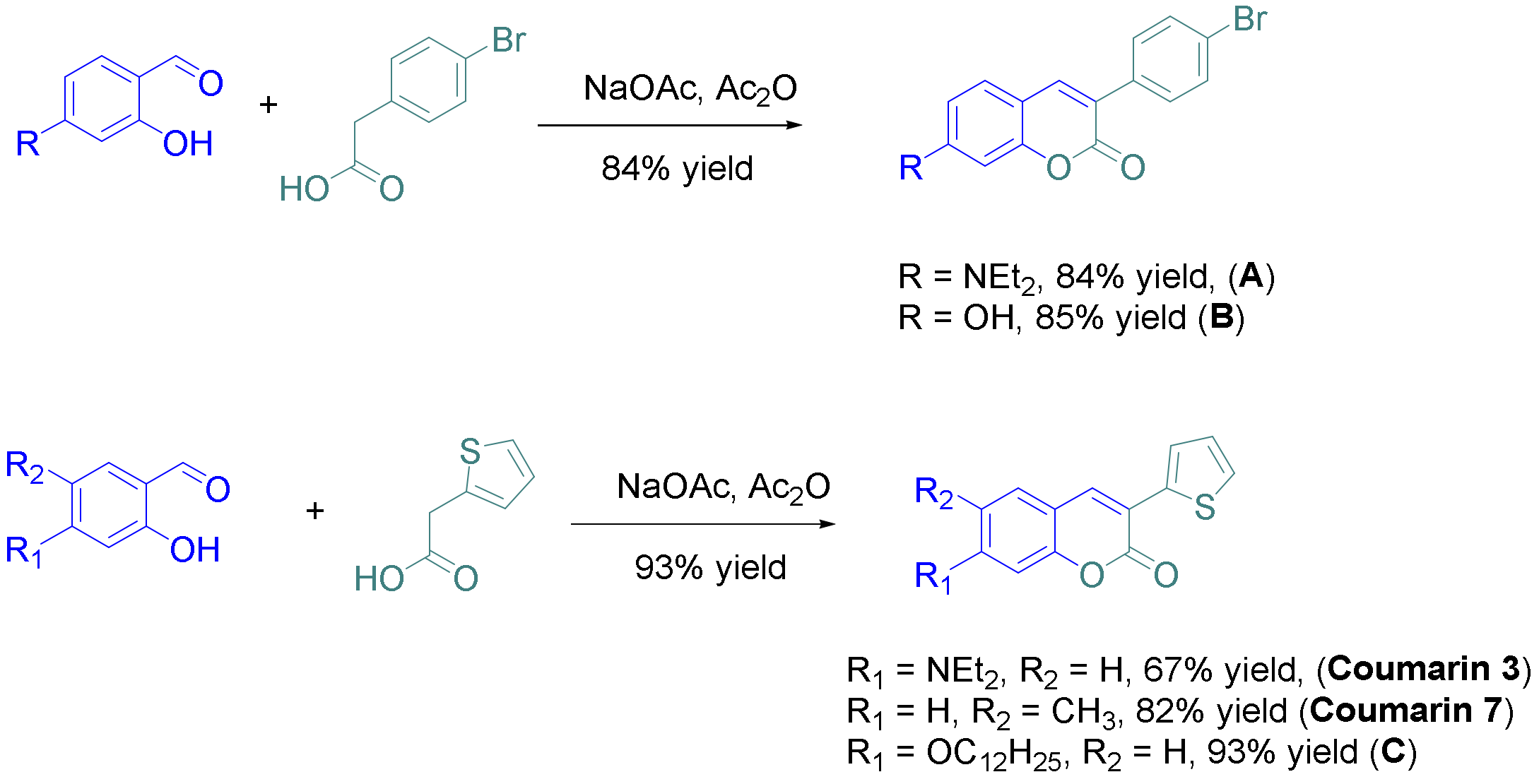
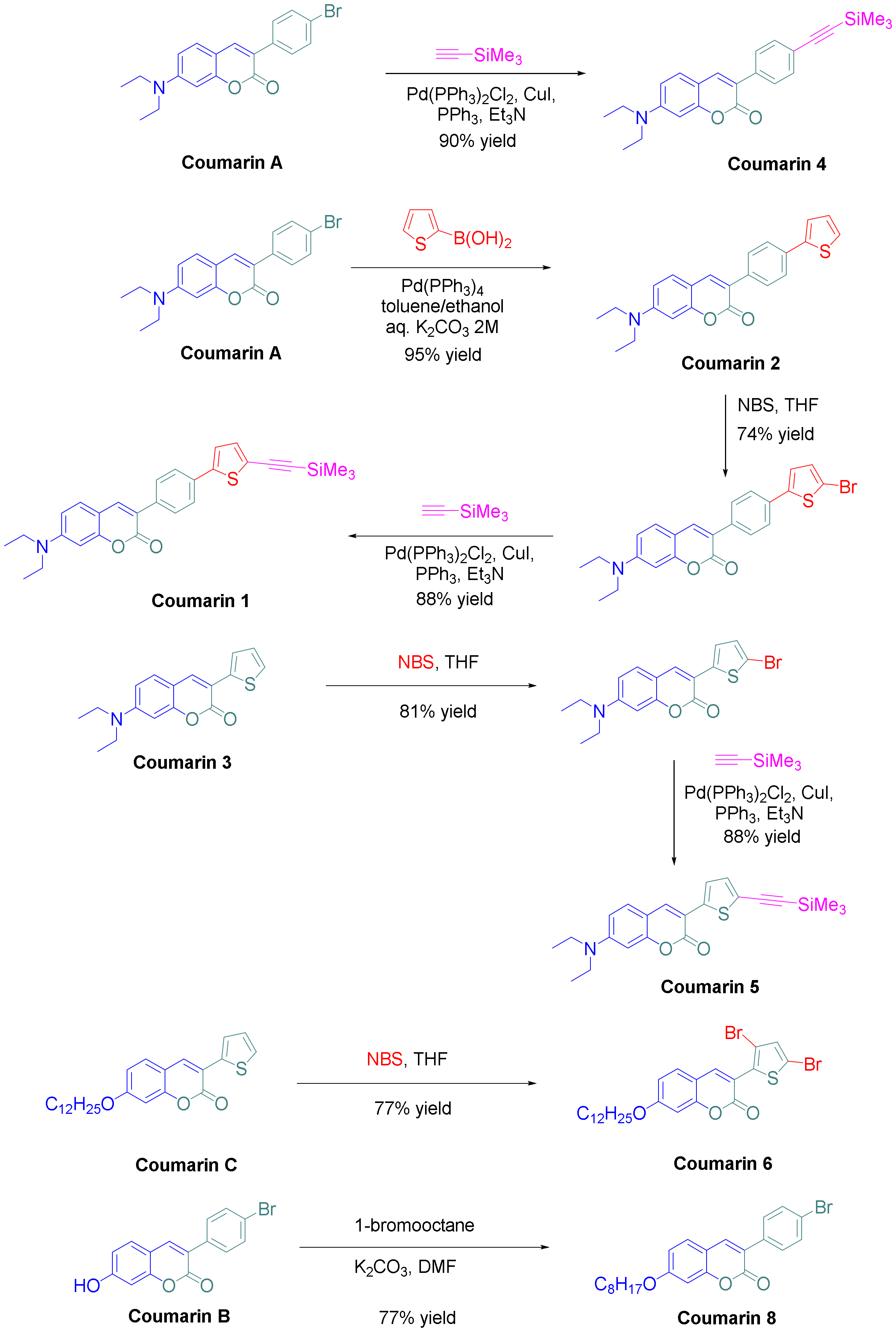
2.1. Synthesis of Coumarins
2.2. Light Absorption Properties of the Examined Coumarins
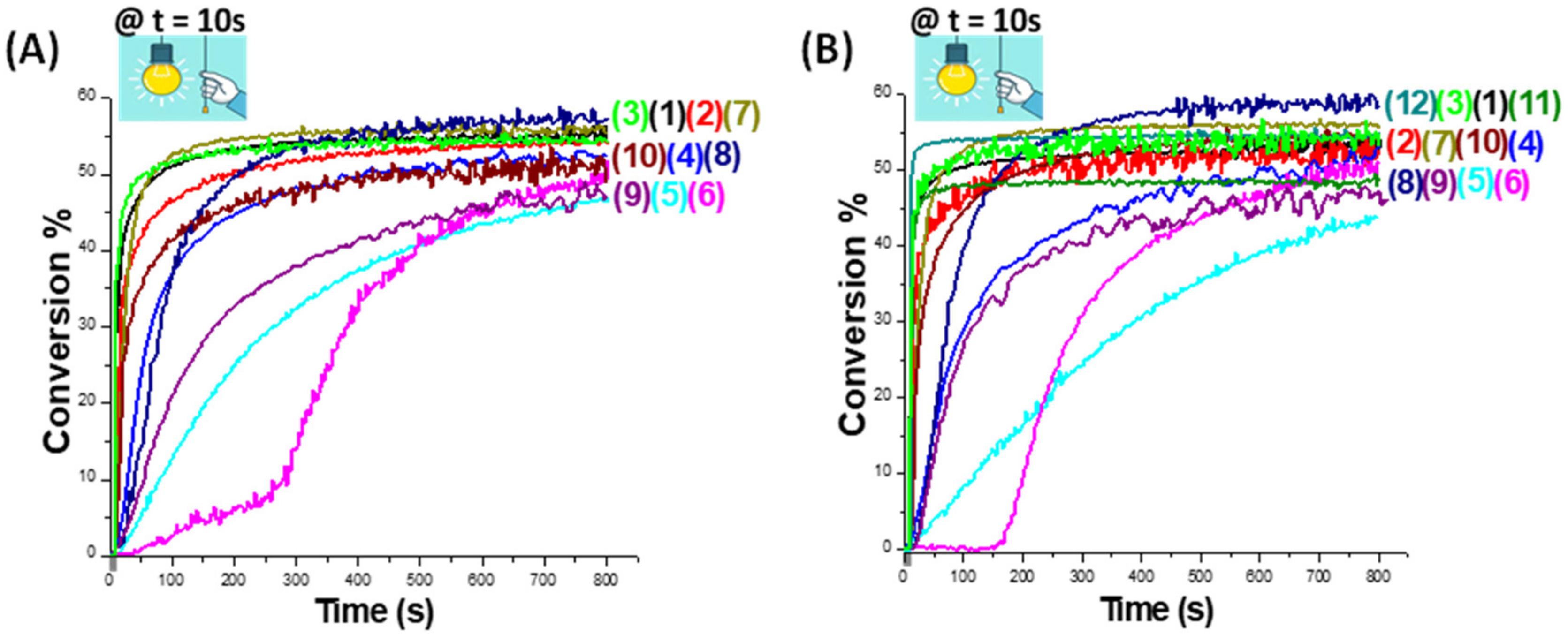
2.3. Cationic Photopolymerization (CP) of Epoxy-Silicones
2.4. 3D Patterns in the Presence of Coumarin/IOD Couples
3. Materials and Methods
3.1. Synthesis of the Coumarins Investigated in This Research
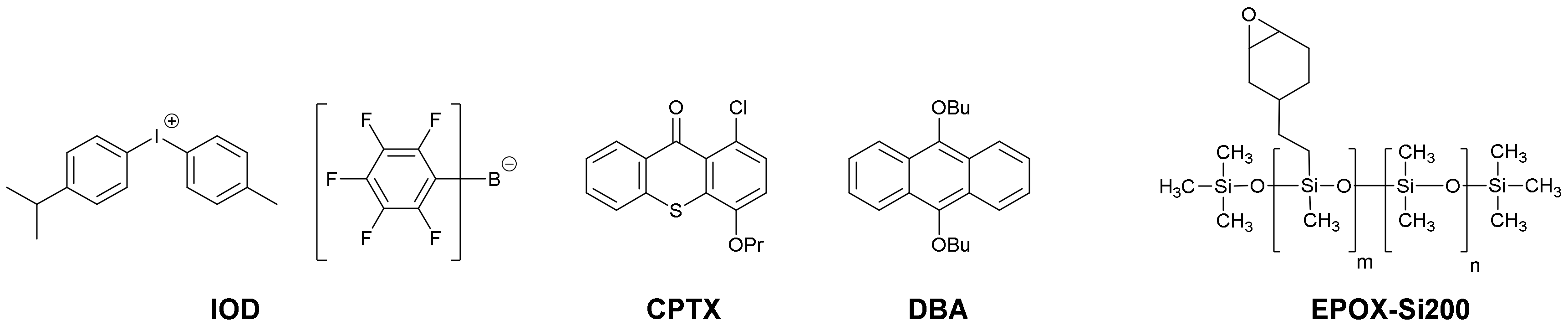
3.2. Commercial Chemical Compounds Used in This Study
3.3. Light Irradiation Sources
3.4. Cationic Photopolymerization (CP) Followed by Real-Time Fourier Transform InfraRed Spectroscopy (RT-FTIR)
3.5. Redox Potentials
3.6. UV-Visible Absorption Experiments
3.7. Fluorescence Experiments
3.8. 3D Printing Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steindl, J.; Svirkova, A.; Marchetti-Deschmann, M.; Moszner, N.; Gorsche, C. Light-Triggered Radical Silane-Ene Chemistry Using a Monosubstituted Bis(Trimethylsilyl)Silane. Macromol. Chem. Phys. 2017, 218, 1600563. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gu, M. Decomposition Kinetics, Life Estimation, and Dielectric Study of an Acrylate Based Photopolymer for Microfabrication and Photonic Applications. Macromol. Chem. Phys. 2005, 206, 1659–1664. [Google Scholar] [CrossRef]
- Tang, C.; Liu, W. Synthesis of Cationic UV-Curable Methacrylate Copolymers and Properties of the Cured Films of Their Composites with Alicyclic Epoxy Resin. J. Appl. Polym. 2012, 123, 1724–1731. [Google Scholar] [CrossRef]
- Balakrishnan, P.S.; Murugavel, S.C. Spectral, Thermal, and Photoreactivity Studies on Epoxy Resin Containing Benzylidene Units in the Main Chain. J. Appl. Polym. 2009, 111, 2340–2344. [Google Scholar] [CrossRef]
- Crivello, J.V.; Ortiz, R.A. Design and Synthesis of Highly Reactive Photopolymerizable Epoxy Monomers. J. Polym. Sci. Part. A: Pol. Chem. 2001, 39, 2385–2395. [Google Scholar] [CrossRef]
- Sangerano, M.; Pallaro, E.; Roppolo, I.; Rizza, G. UV-Cured Epoxy Coating Reinforced with Sepiolite as Inorganic Filler. J. Mater. Sci. 2009, 44, 3165–3171. [Google Scholar] [CrossRef]
- Sangermano, M.; Tonin, M.; Yagci, Y. Degradable Epoxy Coatings by Photoinitiated Cationic Copolymerization of Bisepoxide with ε-Caprolactone. Eur. Polym. 2010, 46, 254–259. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. A Breakthrough toward Long Wavelength Cationic Photopolymerization: Initiating Systems Based on Violanthrone Derivatives and Silyl Radicals. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Combination of Transition Metal Carbonyls and Silanes: New Photoinitiating Systems. J. Polym. Sci. Part. A: Pol. Chem. 2010, 48, 1830–1837. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, L.; Lu, C.; Teng, S.; Young, M.W.; Gogos, C.G. A New Fluidized Bed Coating Process via Photo-Initiated Cationic Polymerization. Polym. Eng. Sci. 2009, 49, 1107–1116. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Photopolymerization of N-Vinylcarbazole Using Visible-Light Harvesting Iridium Complexes as Photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Liow, S.S.; Lipik, V.T.; Widjaja, L.K.; Abadie, M.J.M. Synthesis, Characterization and Photopolymerization of Vinyl Ether and Acrylate Functionalized Hybrid Oligo-Caprolactone. J. Polym. Res. 2011, 19, 9748. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin Derivatives as Versatile Photoinitiators for 3D Printing, Polymerization in Water and Photocomposite Synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Abdallah, M.; Dumur, F.; Hijazi, A.; Rodeghiero, G.; Gualandi, A.; Cozzi, P.G.; Lalevée, J. Keto-coumarin Scaffold for Photoinitiators for 3D Printing and Photocomposites. J. Polym. Sci. 2020, 10, 872–884. [Google Scholar] [CrossRef]
- Abdallah, M.; Magaldi, D.; Hijazi, A.; Graff, B.; Dumur, F.; Fouassier, J.P.; Bui, T.T.; Goubard, F.; Lalevée, J. Development of new high-performance visible light photoinitiators based on carbazole scaffold and their applications in 3d printing and photocomposite synthesis. J. Polym. Sci. Part. A: Pol. Chem. 2019, 54, 2081–2092. [Google Scholar] [CrossRef]
- Abdallah, M.; Le, H.; Hijazi, A.; Schmitt, M.; Graff, B.; Dumur, F.; Bui, T.T.; Goubard, F.; Fouassier, J.P.; Lalevée, J. Acridone derivatives as high performance visible light photoinitiators for cationic and radical photosensitive resins for 3D printing technology and for low migration photopolymer property. Polymer 2018, 159, 47–58. [Google Scholar] [CrossRef]
- Abdallah, M.; Bui, T.T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.P.; Lalevée, J. Phenothiazine Derivatives as Photoredox Catalysts for Cationic and Radical Photosensitive Resins for 3D Printing Technology and Photocomposite Synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bi, D. Regioselective hydrosilations. IV. The synthesis and polymerization of monomers containing epoxy and alkoxysilane groups. J. Polym. Sci. Part. A: Pol. Chem. 1993, 31, 3121–3132. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. The synthesis, characterization, and photoinitiated cationic polymerization of silicon-containing epoxy resins. J. Polym. Sci. Part. A: Pol. Chem. 1990, 28, 479–503. [Google Scholar] [CrossRef]
- Upul Ranaweera, R.A.A.; Schuman, T.P.; Wang, R.; Miller, B.D.; Kilway, K.V. Effect of moisture on cationic polymerization of silicone epoxy monomers. J. Appl. Polym. 2015, 132, 41831. [Google Scholar] [CrossRef]
- Koliniotou-Koumpia, E.; Kouros, P.; Dionysopoulos, D.; Zafiriadis, L. Bonding strength of silorane-based composite to Er-YAG laser prepared dentin. Lasers Med. Sci. 2015, 30, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ Printing: Computer-aided jet-based 3D tissue engineering. Biotechnol. J. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J.; et al. Clinical Applications of 3D Printing: Primer for Radiologists. Acad. Radiol. 2018, 25, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Prahadeesh, N.; Sithambaresan, M.; Mathiventhan, U. A Study on Hydrogen Peroxide Scavenging Activity and Ferric Reducing Ability of Simple Coumarins. Emerg. Sci. J. 2018, 2, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Han Ahn, K.; Gryko, D.T. π-Expanded coumarins: Synthesis, optical properties and applications. J. Mater. Chem. C. 2015, 3, 1421–1446. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Photocatalysts in Polymerization Reactions. ChemCatChem 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Budreckiene, R.; Lazauskaite, R.; Buika, G.; Grazulevicius, J.V. Cationic photopolymerization of carbazolyl-containing vinyl ethers. J. Photochem. Photobio. A Chem. 2003, 157, 117–123. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis, Scope, Reactivity, and Efficiency; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Green Bulb Light Source Induced Epoxy Cationic Polymerization under Air Using Tris(2,2′-bipyridine)ruthenium(II) and Silyl Radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient Dual Radical/Cationic Photoinitiator under Visible Light: A New Concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A.A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structure Design of Naphthalimide Derivatives: Toward Versatile Photoinitiators for Near-UV/Visible LEDs, 3D Printing, and Water-Soluble Photoinitiating Systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Copper Complexes in Radical Photoinitiating Systems: Applications to Free Radical and Cationic Polymerization upon Visible LEDs. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
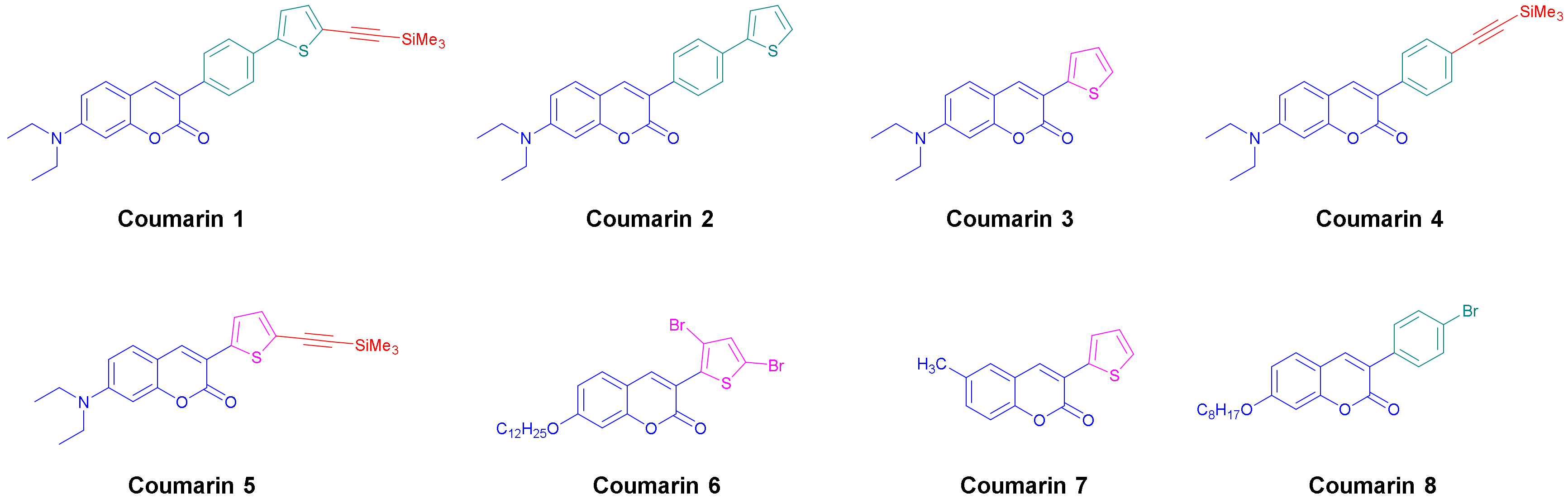
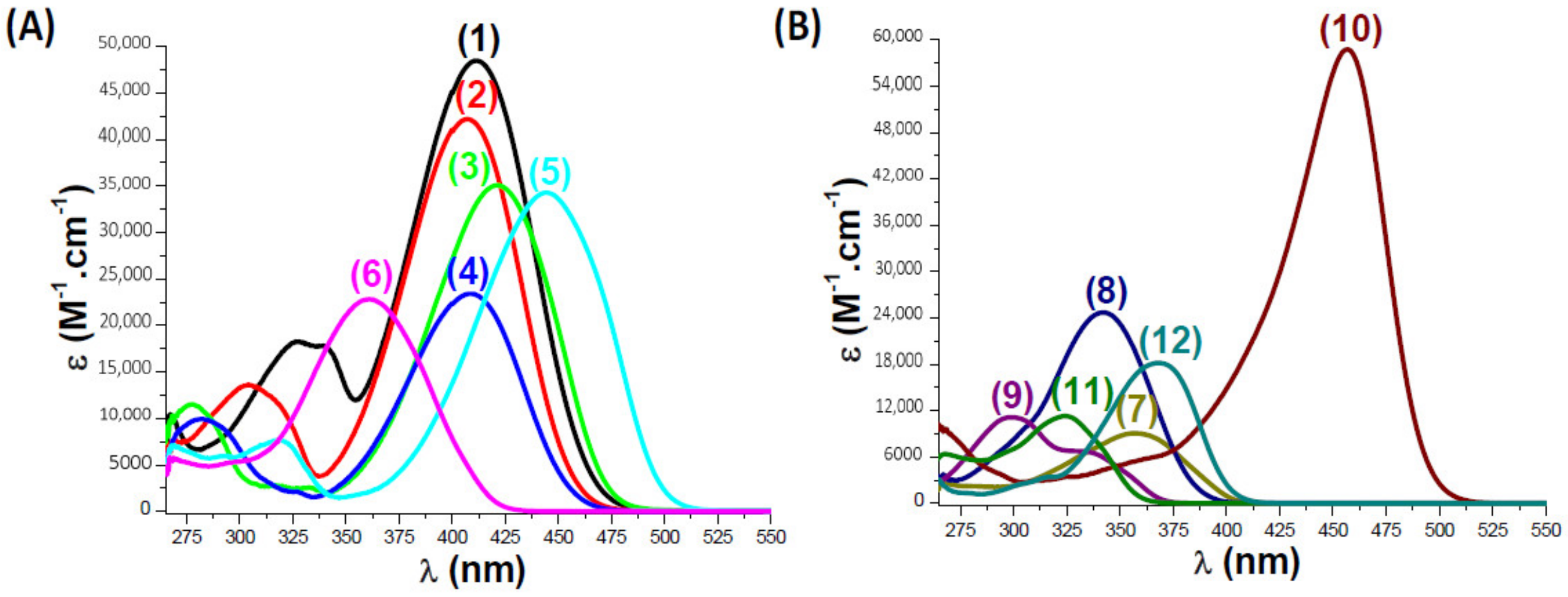
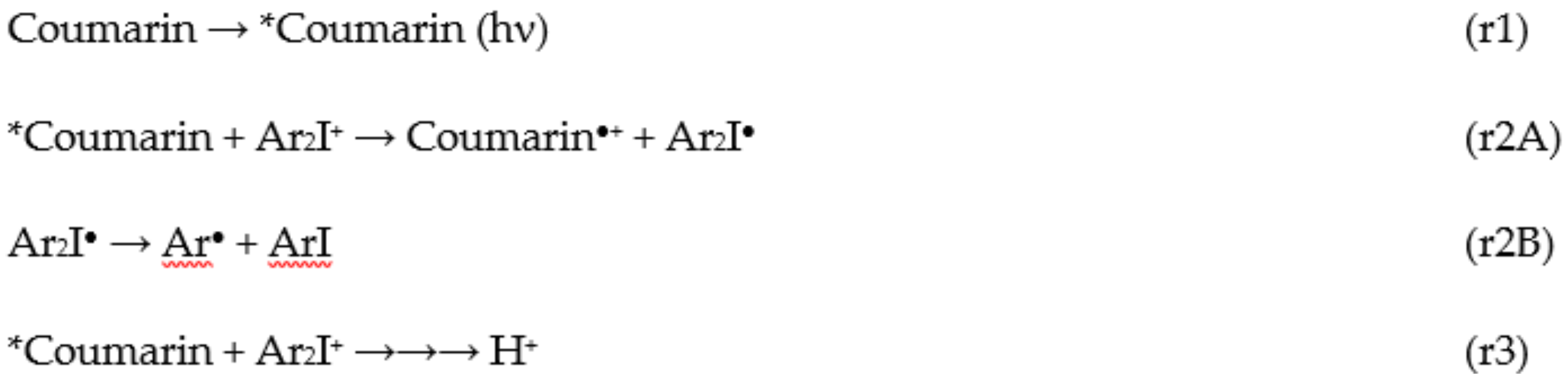


| PI | λmax (nm) | εmax (M−1. cm−1) | ε@405 nm (M−1. cm−1) |
|---|---|---|---|
| Coumarin 1 | 411 | 48630 | 47950 |
| Coumarin 2 | 407 | 42360 | 42270 |
| Coumarin 3 | 421 | 35200 | 30600 |
| Coumarin 4 | 408 | 23530 | 23340 |
| Coumarin 5 | 445 | 34380 | 16880 |
| Coumarin 6 | 361 | 22970 | 6080 |
| Coumarin 7 | 357 | 9210 | 530 |
| Coumarin 8 | 342 | 24760 | 260 |
| Com. Coum 1 | 299 | 11150 | 40 |
| Com. Coum 2 | 457 | 58640 | 17450 |
| Com. Coum 3 | 324 | 11310 | 40 |
| Com. Coum 4 | 368 | 18200 | 1100 |
| Coumarin 1 | Coumarin 2 | Coumarin 3 | Coumarin 4 | Coumarin 5 | Coumarin 6 | Coumarin 7 | Coumarin 8 | Com. Coum 2 | Com. Coum 4 | CPTX | DBA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 57% a | 55% a | 55% a | 53% a | 47% a | 52% a | 57% a | 59% a | 47% a | 52% a | 48% b | 55% b |
| 55% b | 55% b | 55% b | 54% b | 44% b | 52% b | 57% b | 60% b | 47% b | 55% b |
| PI | ES1 (eV) | ET1 (eV)a | Eox (eV) | ΔGet(S1)b(PI/Iod) (eV) | ΔGet(T1)b(PI/Iod) (eV) |
|---|---|---|---|---|---|
| Coumarin 1 | 2.7 | 1.94 | 1.03 | −1.47 | −0.71 |
| Coumarin 2 | 2.74 | 1.99 | 0.95 | −1.59 | −0.84 |
| Coumarin 3 | 2.68 | 1.81 | 0.81 | −1.67 | −0.8 |
| Coumarin 4 | 2.74 | 2.04 | 0.35 | −2.19 | −1.49 |
| Coumarin 5 | 2.55 | 1.67 | 0.45 | −1.9 | −1.02 |
| Coumarin 6 | 3.02 | 1.91 | 0.47 | −2.35 | −1.24 |
| Coumarin 7 | 2.69 | 1.81 | 1.12 | −1.37 | −0.49 |
| Coumarin 8 | 3.22 | 2.19 | 0.45 | −2.57 | −1.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.; Hijazi, A.; Dumur, F.; Lalevée, J. Coumarins as Powerful Photosensitizers for the Cationic Polymerization of Epoxy-Silicones under Near-UV and Visible Light and Applications for 3D Printing Technology. Molecules 2020, 25, 2063. https://doi.org/10.3390/molecules25092063
Abdallah M, Hijazi A, Dumur F, Lalevée J. Coumarins as Powerful Photosensitizers for the Cationic Polymerization of Epoxy-Silicones under Near-UV and Visible Light and Applications for 3D Printing Technology. Molecules. 2020; 25(9):2063. https://doi.org/10.3390/molecules25092063
Chicago/Turabian StyleAbdallah, Mira, Akram Hijazi, Frédéric Dumur, and Jacques Lalevée. 2020. "Coumarins as Powerful Photosensitizers for the Cationic Polymerization of Epoxy-Silicones under Near-UV and Visible Light and Applications for 3D Printing Technology" Molecules 25, no. 9: 2063. https://doi.org/10.3390/molecules25092063






