Synthesizing of Novel Bulk (Zr67Cu33)100−xWx(x; 5–30 at%) Glassy Alloys by Spark Plasma Sintering of Mechanically Alloyed Powders
Abstract
:1. Introduction
2. Results and Discussion
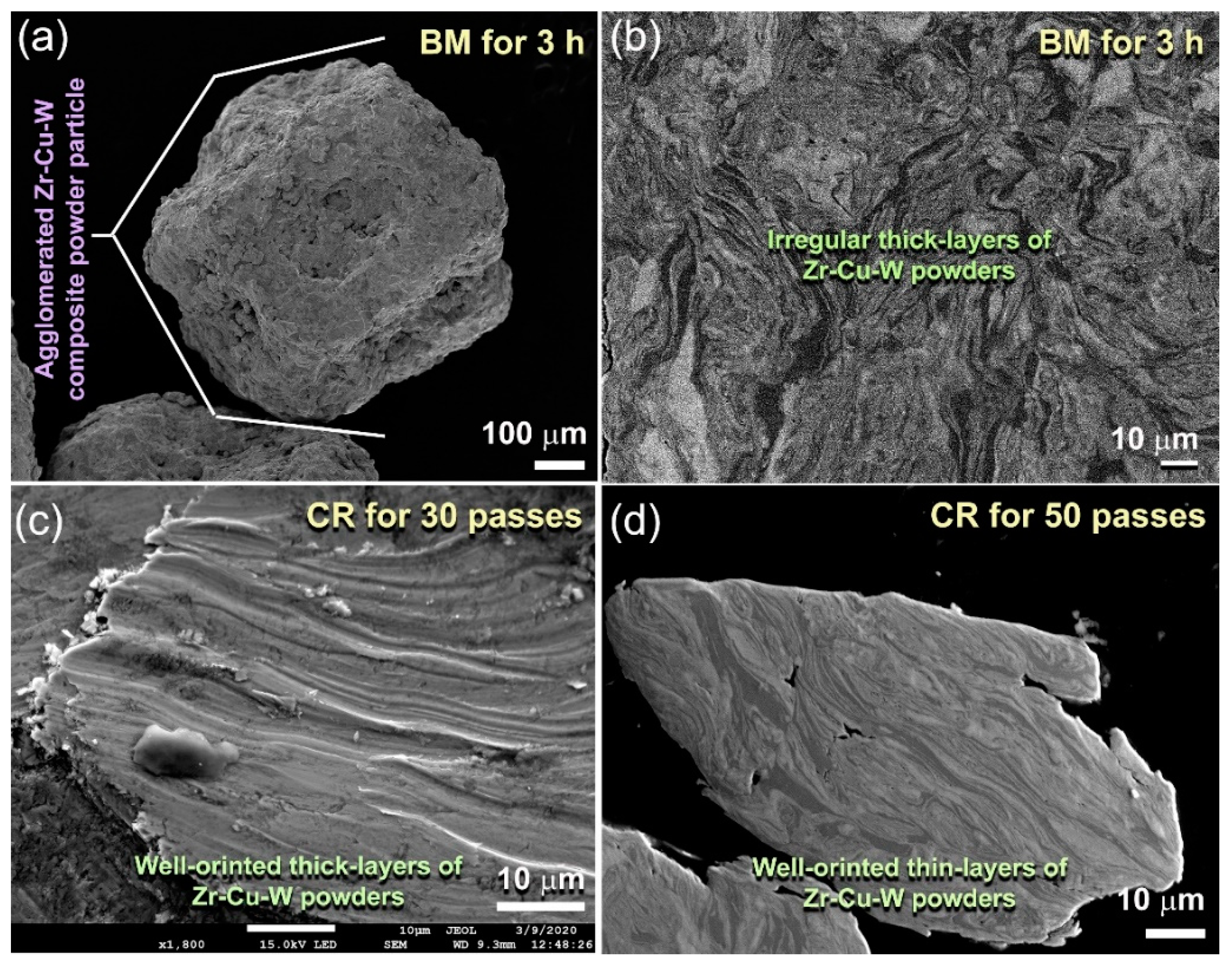
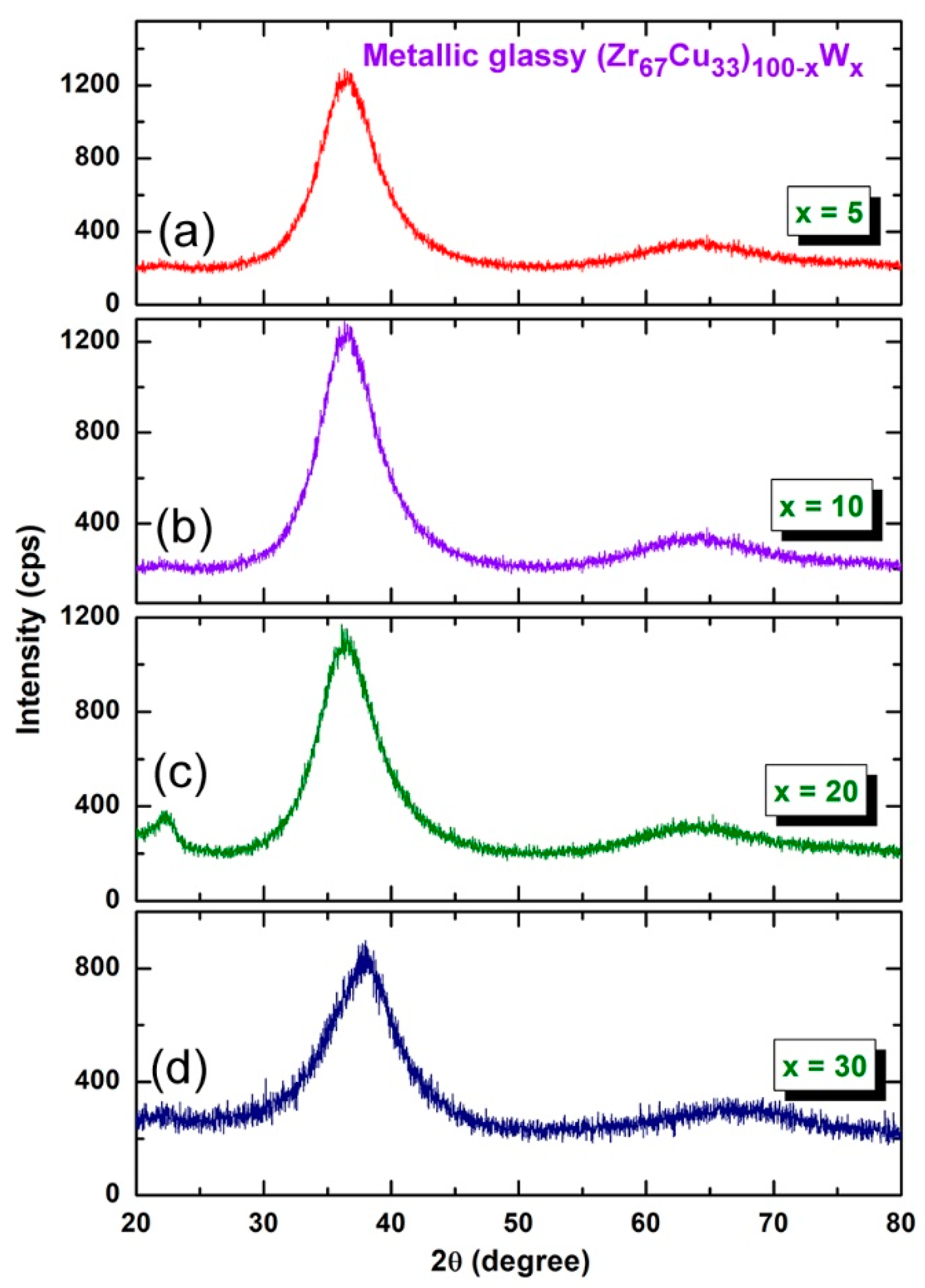
2.1. Morphological and Structural Changes with Changing the CR and BM Times
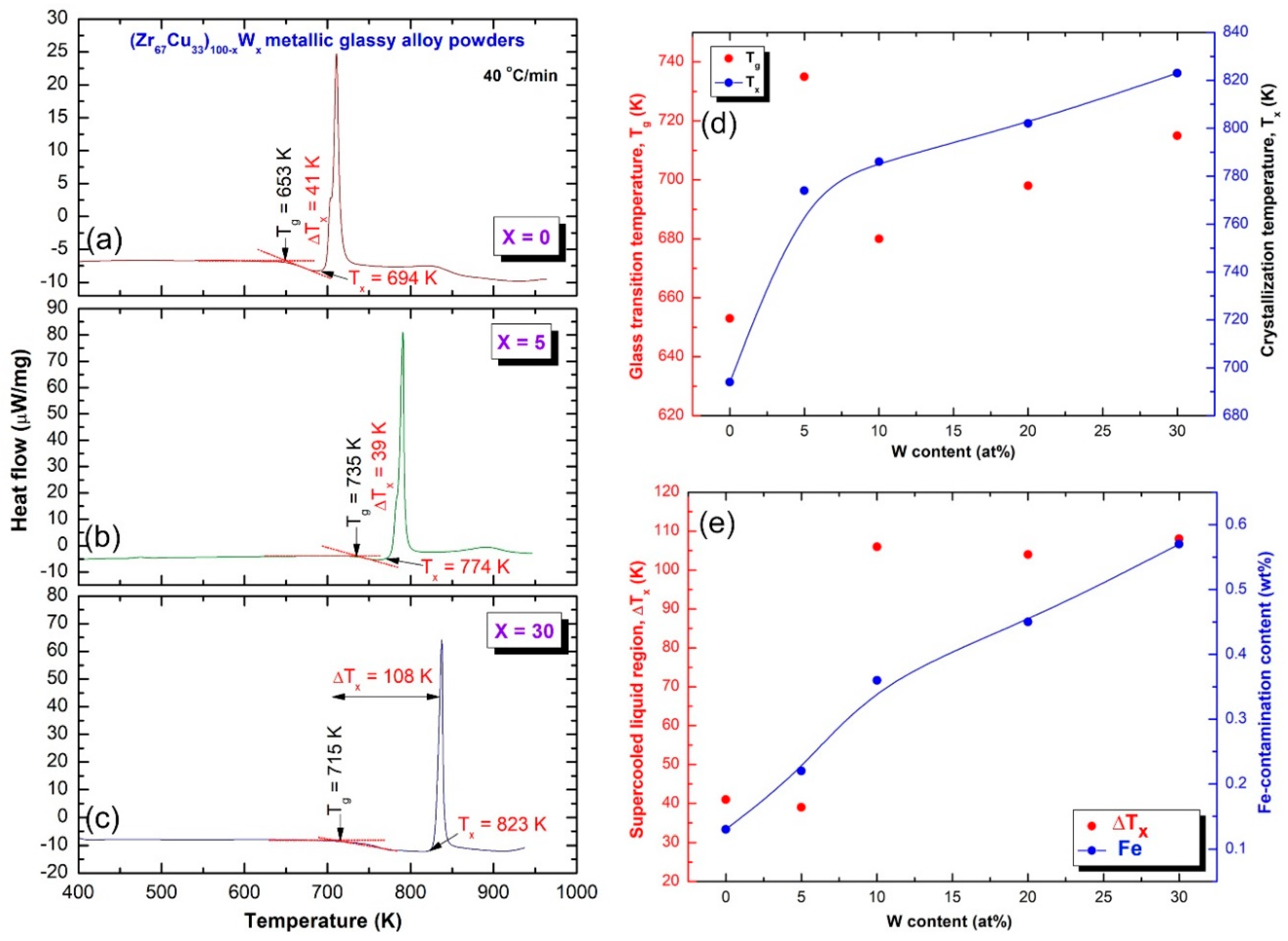
2.2. Thermal Stability
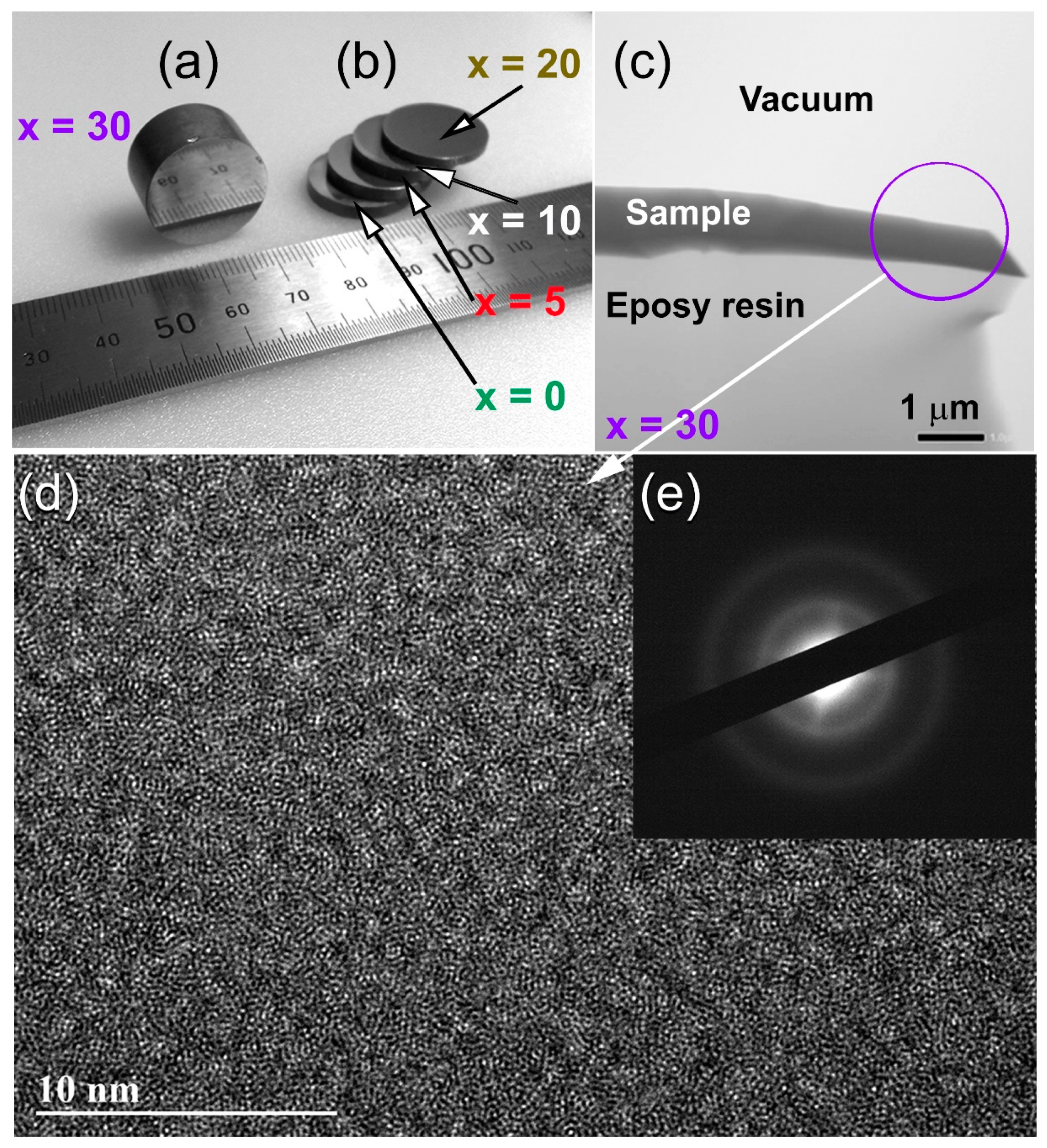
2.3. Fabrication of Bulk Metallic-Glassy (Z67Cu33)100−xWx Alloys by SPS Technique
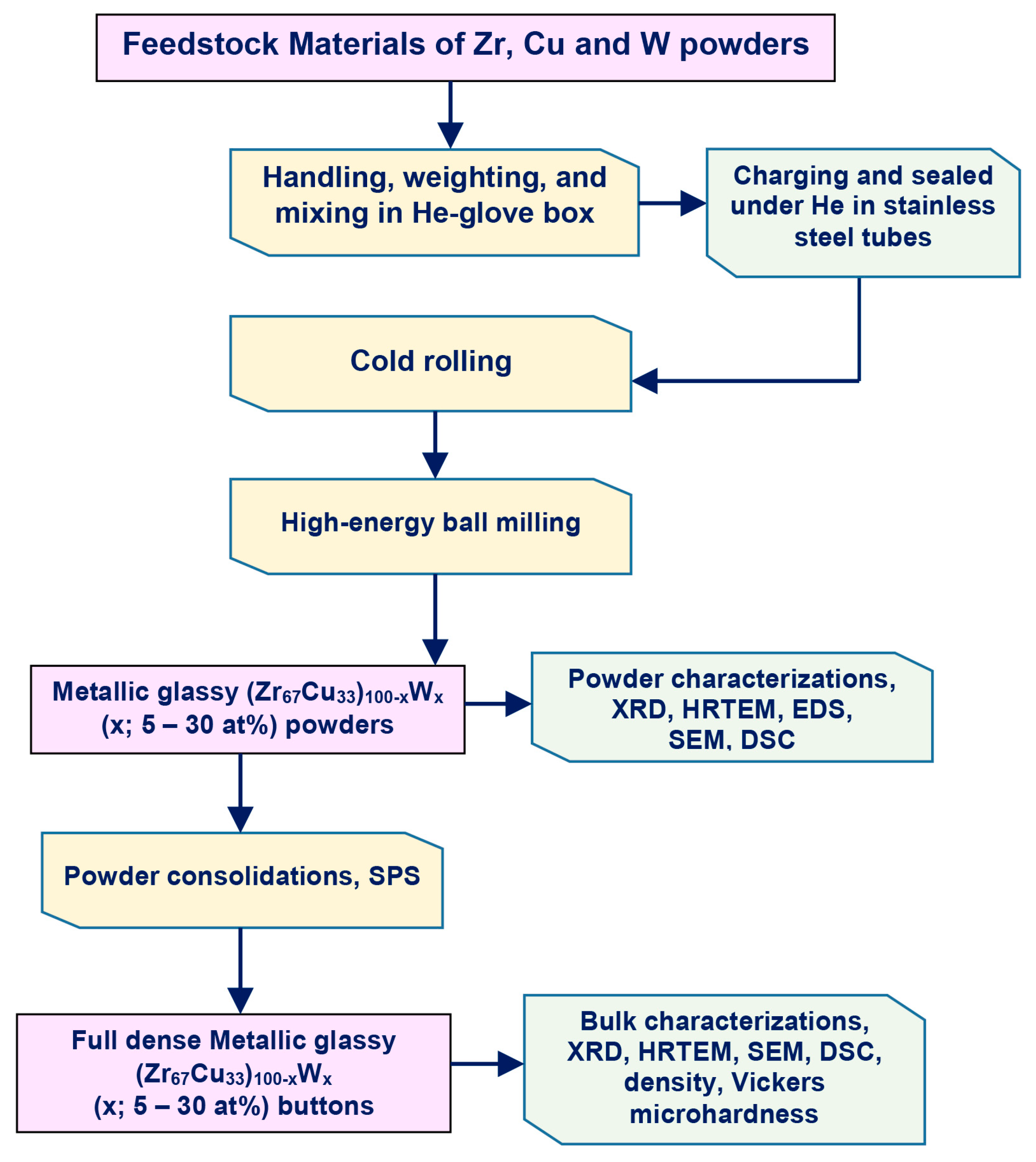
3. Materials and Methods
3.1. Feedstock Materials
3.2. Cold Rolling and High-Energy Ball Milling Procedures
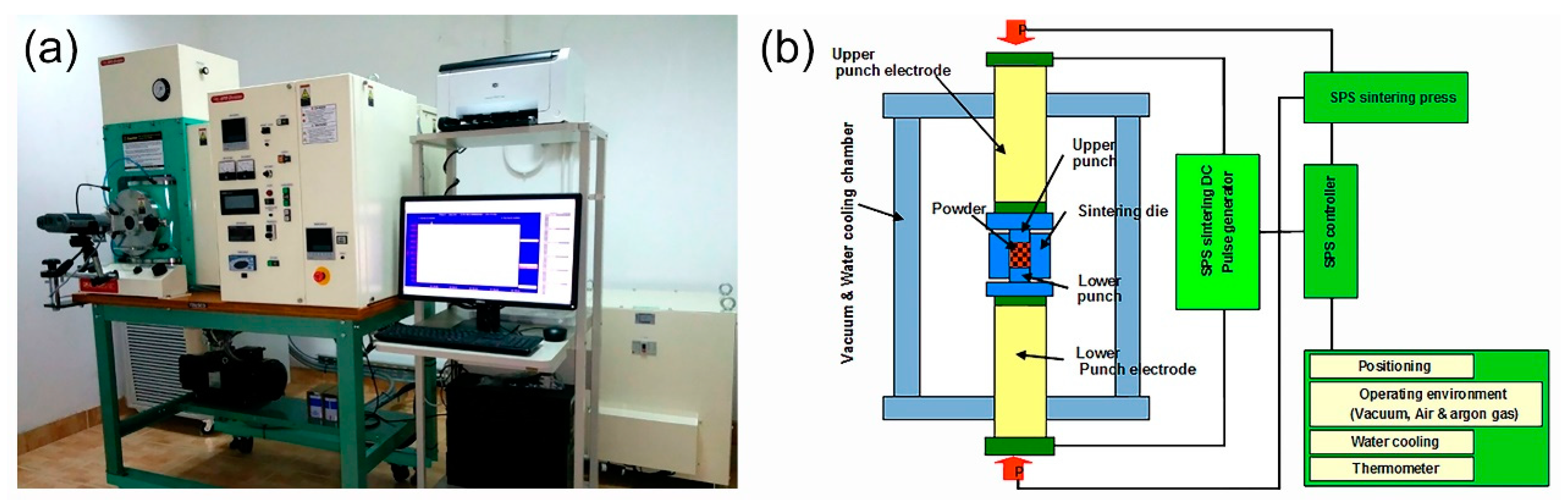
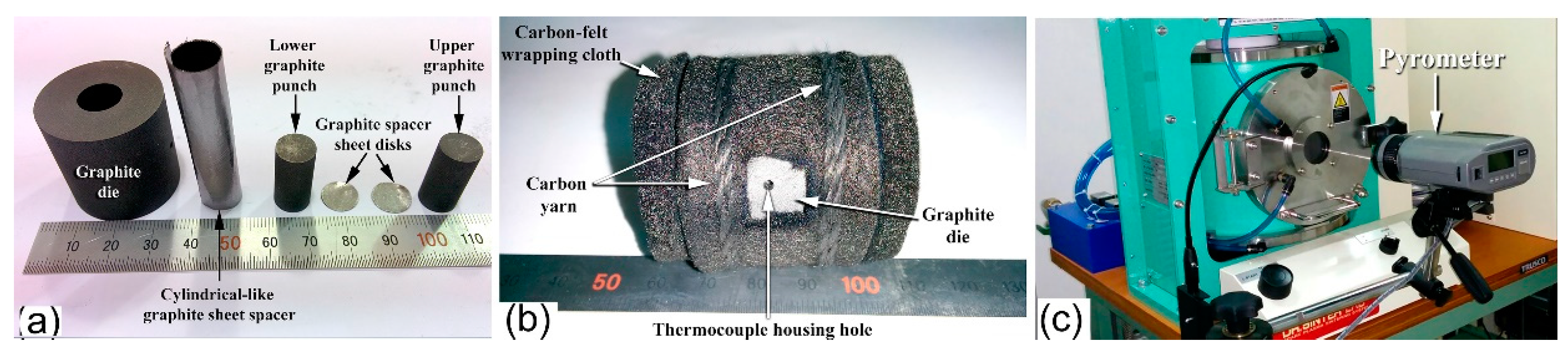
3.3. Powder Consolidation by Spark Plasma Sintering (SPS)
3.4. Sample Characterizations
3.4.1. Crystal Structure
3.4.2. Morphology and Elemental Analysis
3.4.3. Thermal Stability
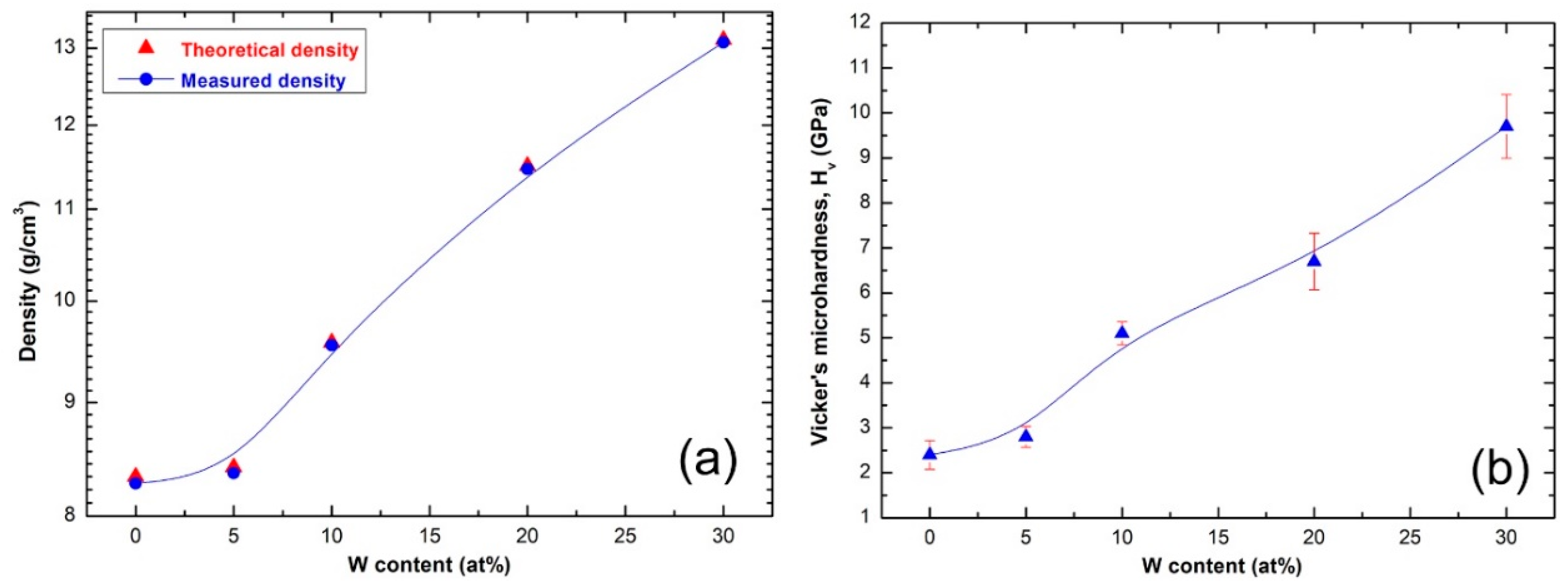
3.4.4. Density and Vickers Microhardness
4. Conclusions
- (1)
- The system can be obtained successfully in wide W concentrations, extended from 5 to 30 at%.
- (2)
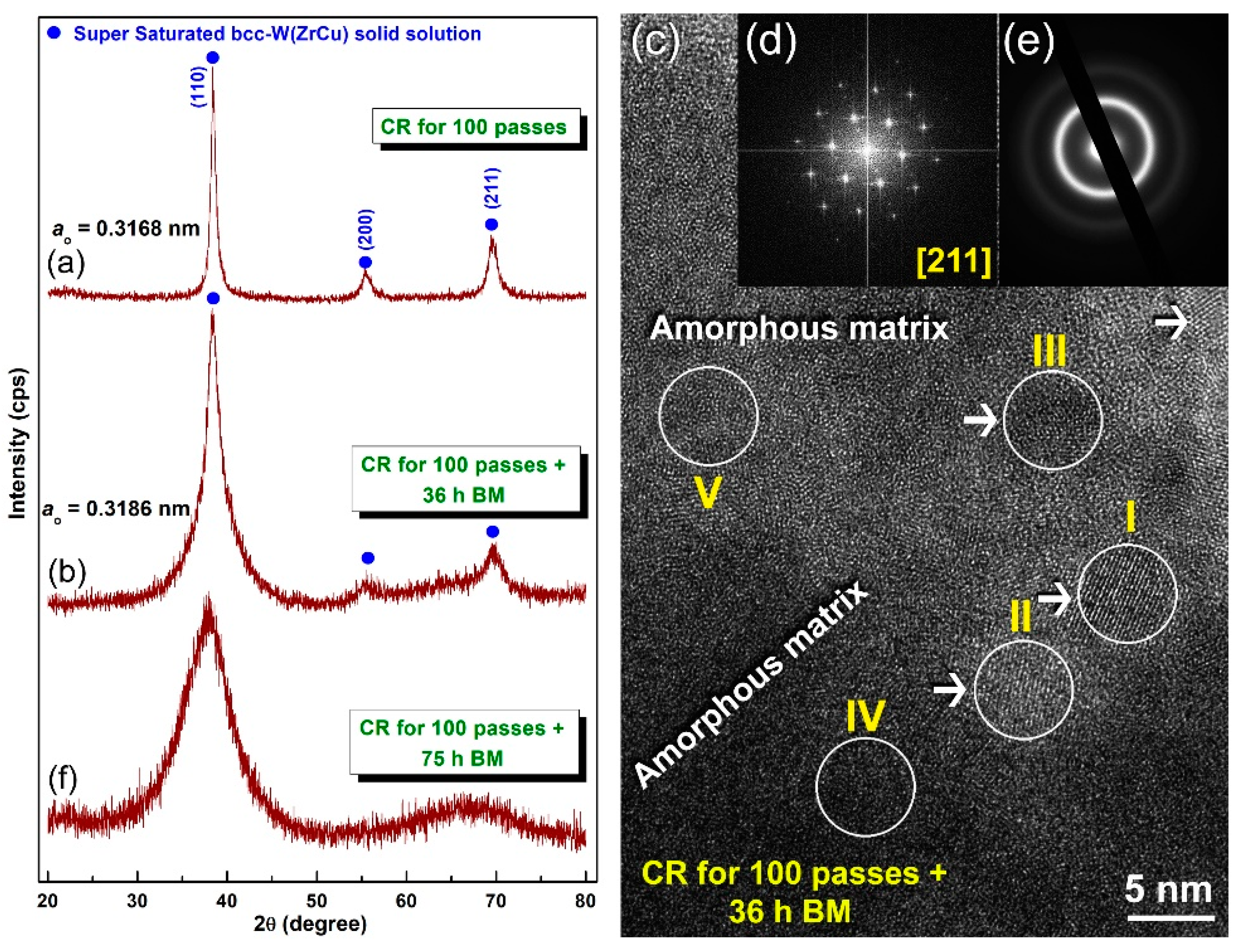
- Pretreatment of the feedstock Zr, Cu, and W metal powders, using the cold rolling method, led to obtaining well-aligned multilayered structure particles. Increasing the cold rolling time to 100 passes enhanced the solid-state diffusion between Zr/Cu/W layered and led to obtaining a supersaturated solid-solution phase.
- (3)
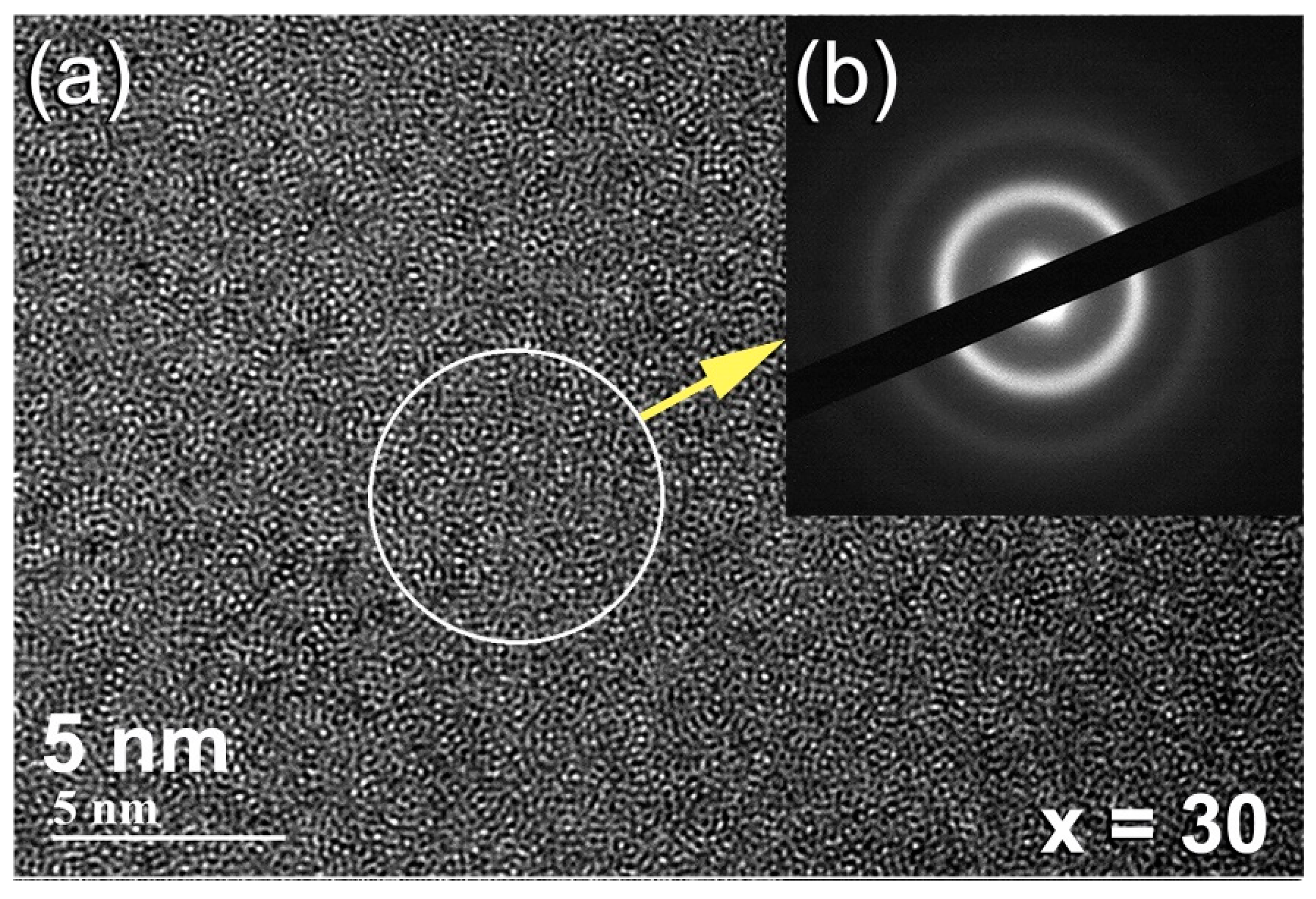
- When the solid solution powders were subjected to high-energy ball milling for 75 h, the bcc-solid solution phase could not withstand the severe plastic deformation and imperfections generated by the balls milling media and transformed into a metallic glassy phase.
- (4)
- The as-fabricated (Zr67Cu33)100−xWx metallic glassy alloys revealed excellent GFA and good thermal stability, as indicated by their wide ∆Tx, and high Tx values.
- (5)
- Based on the their wide ∆Tx before crystallizations and high Tx, the as-fabricated powders were consolidated into BMG buttons, while using SPS technique.
- (6)
- The SPS consolidation step maintained the original short-range order structure after consolidation without experiencing any partial crystallizations.
- (7)
- The metallic glassy consolidated buttons were nearly full dense (above 99.95%).
- (8)
- The Vickers microhardness have shown a monotonical increase (from 2.8 ± 0.23 GPa to 9.7 ± 0.71 GPa Hv), depending on the W contents (5 at% to 30 at%).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Eskandarany, M.S.; Saida, J.; Inoue, A. Room-temperature mechanically induced solid state devitrifications of glassy Zr65Al7.5Ni10Cu12.5Pd5 alloy powders. Acta Mater. 2003, 51, 4519–4532. [Google Scholar] [CrossRef]
- Duwez, P.; Willens, R.H.; Klement, W., Jr. Continuous series of metastable solid solutions in silver-copper alloys. J. Appl. Phys. 1960, 31, 1136–1137. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying for Fabrication of Advanced Engineering Materials, 1st ed.; Elsevier: Oxford, UK, 2001; pp. 142–148. [Google Scholar]
- Lenain, A.; Blandin, J.; Kapelski, G.; Volpi, F.; Gravier, S. Hf-rich bulk metallic glasses as potential insulating structural material. Mater. Design. 2018, 139, 467–472. [Google Scholar] [CrossRef]
- Wang, W.H. Dynamic relaxations and relaxation-property relationships in metallic glasses. Prog. Mater. Sci. 2019, 106, 100561. [Google Scholar] [CrossRef]
- Ford, D.C.; Hicks, D.; Oses, C.; Toher, C.; Curtarolo, S. Metallic glasses for biodegradable implants. Acta Mater. 2019, 176, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Louzguine-Luzgin, D.V.; Bazlov, A.; Ketov, S.; Greer, A.L.; Inoue, A. Crystal growth limitation as a critical factor for formation of Fe-based bulk metallic glasses. Acta Mater. 2015, 176, 396–402. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses, 1st ed.; CRC Press, Taylor & Francis Group: Abingdon-on-Thames, UK, 2011; pp. 11–40. [Google Scholar]
- El-Eskandarany, M.S.; Inoue, A. Phys. Rev. B 2007, 75, 224109. [CrossRef]
- El-Eskandarany, M.; Aoki, K.; Sumiyama, K.; Suzuki, K. Cyclic phase transformations of mechanically alloyed Co75Ti25 powders. Acta Mater. 2002, 50, 1113–1123. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Inoue, A. Synthesis of new bulk metallic glassy Ti60Al15Cu10W10Ni5 alloy by hot pressing the mechanically alloyed powders at the supercooled liquid region. Met. Trans. A 2006, 37, 2231–2238. [Google Scholar] [CrossRef]
- Lin, W.-H.; Teng, Y.; Sha, Z.-D.; Yuan, S.-Y.; Branicio, P.S. Mechanical properties of nanoporous metallic glasses: Insights from large-scale atomic simulations. Int. J. Plast. 2020, 127, 102657. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying. In Energy Storage, Protective Coatings, and Medical Applications, 3rd ed.; Elsevier: Oxford, UK, 2020; in press. [Google Scholar]
- Inoue, A.; Kong, F.; Han, Y.; Zhu, S.; Churyumov, A.; Shalaan, E.; Al-Marzouki, F. Development and application of Fe-based soft magnetic bulk metallic glassy inductors. J. Alloys Compd. 2018, 731, 1303–1309. [Google Scholar] [CrossRef]
- Yuan, C.; Lv, Z.; Pang, C.; Zhu, W.; Wang, X.-L.; Shen, B. Pronounced nanoindentation creep deformation in Cu-doped CoFe-based metallic glasses. J. Alloys Compd. 2019, 806, 246–253. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, B.; Jin, Z.; Gao, H.; Ma, M.; Zhang, X. Crystallization kinetics and mechanical properties of Zr56Cu24Al9Ni7-xTi4Agx (x = 0, 1, 3, 5, and 7) metallic glasses. J. Alloys Compd. 2019, 806, 246–253. [Google Scholar] [CrossRef]
- Madge, S. Toughness of bulk metallic glasses. Metals 2015, 5, 1279–1305. [Google Scholar] [CrossRef] [Green Version]
- Lai, L.; He, R.; Ding, K.; Liu, T.; Liu, R.; Chen, Y.; Guo, S. Ternary Co-Mo-B bulk metallic glasses with ultrahigh strength and good ductility. J. Non. Cryst. Solids 2019, 524, 119657. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, G.; Zhou, J.; Yuan, C.; Shen, B. Effects of Ni substitution for Fe/Co on mechanical and magnetic properties of Co-based bulk metallic glasses. J. Alloys Compd. 2020, 820, 246–253. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Zhang, W.; Inoue, A. Mechanically induced solid-state reaction for synthesizing of glassy Co75Ti25 soft magnet alloy powders with wide supercooled liquid region. J. Mater. Res. 2002, 17, 2447–2456. [Google Scholar] [CrossRef]
- Sun, B.; Xin, S.; Shen, T. Microstructural origin of the ultra-low coercivity in bulk Fe65.5Cr4Mo4Ga4P12B5.5C5 metallic glasses. J. Magn. Magn. Mater. 2018, 466, 130–132. [Google Scholar] [CrossRef]
- Gu, J.-L.; Shao, Y.; Bu, H.-T.; Jia, J.-L.; Yao, K. An abnormal correlation between electron work function and corrosion resistance in Ti-Zr-Be-(Ni/Fe) metallic glasses. Corros. Sci. 2020, 165, 108392. [Google Scholar] [CrossRef]
- Korkmaz, S.; Afşin Kariper, İ. Glass formation, production and superior properties of Zr-based thin film metallic glasses (TFMGs): A status review. J. Non Cryst. Solids 2020, 527, 15. [Google Scholar] [CrossRef]
- Koch, C.C.; Cavin, O.B.; McKamey, C.G.; Scarbrough, J.O. Preparation of “amorphous” Ni60Nb40 by mechanical alloying. Appl. Phys. Lett. 1983, 43, 1017–1019. [Google Scholar] [CrossRef]
- El-Esksndarany, M.S.; Itoh, F.; Aoki, K.; Suzuki, K. Preparation of AlxTa1-x amorphous alloy powder by mechanical alloying. J. Non Cryst. Solids 1990, 118, 729–732. [Google Scholar] [CrossRef]
- El-Eskandarany, M.; Aoki, K.; Suzuki, K. Calorimetric characterization of the amorphization process for milled Al50Nb50 alloy powders. Scripta Metall. 1991, 25, 1695–1700. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Aoki, K.; Sumiyama, K.; Suzuki, K. Amorphous-crystalline-amorphous transformations of ball-milled aluminum zirconium powder. Met. Trans. A 1999, 30, 1877–1880. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Sumiyama, K.; Suzuki, K. Crystalline-to-amorphous phase transformation in mechanically alloyed Fe50W50 powders. Acta Metall. 1997, 45, 1175–1187. [Google Scholar] [CrossRef]
- Lan, S.; Wu, Z.; Wei, X.; Zhou, J.; Lu, Z.; Neuefeind, J.C.; Wang, X.-L. Structure origin of a transition of classic-to-avalanche nucleation in Zr-Cu-Al bulk metallic glasses. Acta Metall. 2018, 149, 108–118. [Google Scholar] [CrossRef]
- Kumar, G.; Rector, D.; Conner, R.; Schroers, J. Embrittlement of Zr-based bulk metallic glasses. Acta Metall. 2009, 57, 3572–3583. [Google Scholar] [CrossRef]
- Vora, A.M.; Gandhi, A.L. Collective dynamics of Zr-based bulk metallic glasses. Chin. J. Phys. 2019, 62, 284–295. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Qiang, J.; Jiang, H.; Gu, L. Low-cost Zr-based bulk metallic glasses for biomedical devices applications. J. Non. Cryst. Solids 2019, 520, 119442. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying. In Nanotechnology, Materials Science and Powder Metallurgy, 2nd ed.; Elsevier: Oxford, UK, 2015; pp. 228–305. [Google Scholar]
- Li, X.; Yang, C.; Chen, T.; Zhang, L.; Hayat, M.; Cao, P. Influence of powder shape on atomic diffusivity and resultant densification mechanisms during spark plasma sintering. J. Alloys Compd. 2019, 802, 600–608. [Google Scholar] [CrossRef]
- He, G.; Chen, Q. Interpretation of densification behavior of spark plasma sintered Fe-based metallic glass powders from the standpoint of internal friction. J. Alloys Compd. 2019, 797, 213–221. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ishihara, S.; Inoue, A. Mechanism of solid-state reaction for fabrication of new glassy V45Zr22Ni22Cu11 alloy powders and subsequent consolidation. J. Mater. Res. 2003, 18, 2435–2445. [Google Scholar] [CrossRef]
- Tiwari, D.; Basu, B.; Biswas, K. Simulation of thermal and electric field evolution during spark plasma sintering. Ceram. Int. 2009, 35, 699–708. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Hazza, A. Mechanically induced self-propagating reaction and consequent consolidation for the production of fully dense nanocrystalline Ti55C45 bulk material. Mater. Charact. 2014, 97, 92–100. [Google Scholar] [CrossRef]
- Kale, A.B.; Kim, B.-K.; Kim, D.-I.; Castle, E.G.; Reece, M.; Choi, S.-H. An investigation of the corrosion behavior of 316L stainless steel fabricated by SLM and SPS techniques. Mater. Charact. 2020, 163. [Google Scholar] [CrossRef]
- Gong, B.; Yao, T.; Lei, P.; Harp, J.; Nelson, A.T.; Lian, J. Spark plasma sintering (SPS) densified U3Si2 pellets: Microstructure control and enhanced mechanical and oxidation properties. J. Alloys Compd. 2020, 825, 154022. [Google Scholar] [CrossRef]
- Samsonov, G.V. Handbook of the Physicochemical Properties of the Elements, 1st ed.; Springer: New York, NY, USA, 1968; pp. 7–130. [Google Scholar]
- Harris, J.; Benenson, W.; Holger Luts, H.S. Handbook of Physics, 1st ed.; Springer: New York, NY, USA, 2002; pp. 229–251. [Google Scholar]
- Rajabi, M.; Vahidi, M.; Simchi, A.; Davami, P. Effect of rapid solidification on the microstructure and mechanical properties of hot-pressed Al–20Si–5Fe alloys. Mater. Charact. 2009, 60, 1370–1381. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Alloy (SN#) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Nominal Composition (at%) | |||||
| Zr | 67 | 63.65 | 60.3 | 53.6 | 46.9 |
| Cu | 33 | 31.35 | 29.7 | 36.4 | 23.1 |
| W | 0 | 5 | 10 | 20 | 30 |
| Nominal Composition (wt%) | |||||
| Zr | 74.45 | 66.90 | 59.38 | 48.04 | 38.06 |
| Cu | 25.55 | 22.57 | 20.68 | 16.11 | 12.98 |
| W | 0 | 10.53 | 19.94 | 35.85 | 48.96 |
| Real Composition after Processing and Consolidations (wt%) | |||||
| Zr | 74.38 | 67.05 | 59.31 | 48.11 | 37.92 |
| Cu | 25.62 | 22.51 | 20.62 | 16.14 | 12.88 |
| W | 0 | 10.44 | 20.25 | 35.75 | 49.20 |
| Fe-Contamination (that come from the balls) and Oxygen Contents (wt%) | |||||
| Fe | 0.08 | 0.22 | 0.36 | 0.45 | 0.57 |
| Oxygen | 0.16 | 0.13 | 0.26 | 0.22 | 0.18 |
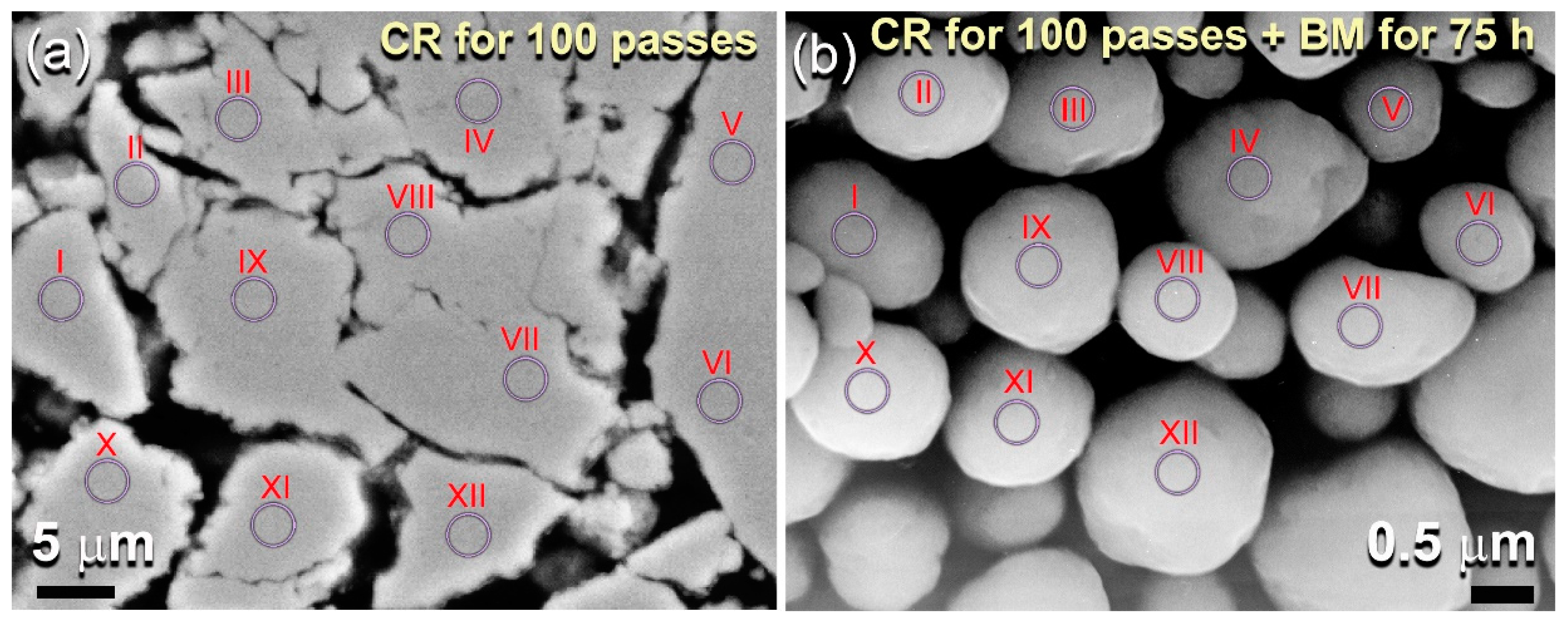
| Alloying Elements (wt%) | ||||
|---|---|---|---|---|
| Zone | Zr | Cu | W | Total |
| (a) CR for 100 Passes | ||||
| I | 37.96 | 13.08 | 48.96 | 100 |
| II | 38.06 | 12.93 | 49.01 | 100 |
| III | 37.94 | 12.89 | 49.17 | 100 |
| IV | 38.09 | 13.02 | 48.89 | 100 |
| V | 37.91 | 13.12 | 48.97 | 100 |
| VI | 38.05 | 12.87 | 49.08 | 100 |
| VII | 38.08 | 12.99 | 48.90 | 100 |
| VIII | 37.98 | 13.02 | 49.00 | 100 |
| IX | 38.05 | 12.93 | 49.02 | 100 |
| X | 38.08 | 13.04 | 48.88 | 100 |
| XI | 37.97 | 12.91 | 49.12 | 100 |
| XII | 38.09 | 13.01 | 48.90 | 100 |
| (b) CR for 100 Passes + BM for 75 h | ||||
| I | 38.02 | 12.93 | 49.05 | 100 |
| II | 38.07 | 12.91 | 49.02 | 100 |
| III | 37.98 | 12.99 | 49.03 | 100 |
| IV | 38.08 | 12.95 | 48.97 | 100 |
| V | 38.07 | 12.97 | 48.96 | 100 |
| VI | 38.01 | 12.96 | 49.03 | 100 |
| VII | 37.97 | 13.06 | 48.97 | 100 |
| VIII | 37.95 | 13.08 | 48.97 | 100 |
| IX | 38.09 | 12.89 | 49.02 | 100 |
| X | 37.98 | 13.01 | 49.01 | 100 |
| XI | 38.09 | 13.03 | 48.88 | 100 |
| XII | 37.99 | 13.03 | 48.98 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Eskandarany, M.S.; Ali, N. Synthesizing of Novel Bulk (Zr67Cu33)100−xWx(x; 5–30 at%) Glassy Alloys by Spark Plasma Sintering of Mechanically Alloyed Powders. Molecules 2020, 25, 1906. https://doi.org/10.3390/molecules25081906
El-Eskandarany MS, Ali N. Synthesizing of Novel Bulk (Zr67Cu33)100−xWx(x; 5–30 at%) Glassy Alloys by Spark Plasma Sintering of Mechanically Alloyed Powders. Molecules. 2020; 25(8):1906. https://doi.org/10.3390/molecules25081906
Chicago/Turabian StyleEl-Eskandarany, M. Sherif, and Naser Ali. 2020. "Synthesizing of Novel Bulk (Zr67Cu33)100−xWx(x; 5–30 at%) Glassy Alloys by Spark Plasma Sintering of Mechanically Alloyed Powders" Molecules 25, no. 8: 1906. https://doi.org/10.3390/molecules25081906






