Abstract
Gymnema sylvestre, a medicinal plant, has been used in Indian ayurvedic traditional medicine for the treatment of diabetes. Phytochemical investigation of Gymnema sylvestre led to the isolation of five new pregnane glycosides, gymsylosides A–E (1–5) and four known oleanane saponins, 3β-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester (6), gymnemoside-W1 (7), 3β-O-β-D-xylopyranosyl-(1→6)-β-D- glucopyranosyl-(1→6)-β-D-glucopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester (8), and alternoside XIX (9). Their structures were identified based on spectroscopic evidence and comparison with those reported in the literature. All compounds were evaluated for their α-glucosidase and α-amylase inhibitory activities. Compounds 2–4 showed significant α-amylase inhibitory activity, with IC50 values ranging from 113.0 to 176.2 µM.
1. Introduction
Gymnema sylvestre (Retz.) R.Br. ex Sm. (Apocynaceae) is a perennial woody climber native to tropical and subtropical regions, such as India, Africa, and southeast Asia. In folk medicine, G. sylvestre have been used to treat snake bites, arthritis, digestive, and enhancing laxative [1,2]. Moreover, the plant has been explored for its benefits in blocking sugar craving and reducing sugar consumption. The recent studies have indicated that G. sylvestre are potential anti-diabetic plants [3,4]. The bioactive components from this plant include pregnane glycosides [5], triterpene saponins [6,7], and flavonoids [8]. Our previous study reported pregnane glycosides from Gymnema inodorum and their α-glucosidase inhibitory activity [9]. As a part of our ongoing investigation on anti-diabetic compounds from Vietnamese plants [10], a methanol extract of the leaves of G. sylvestre was found to inhibit α-glucosidase and α-amylase activities. Herein, we report the isolation, structural elucidation of pregnane-type saponins and oleanane saponins and the evaluation of α-glucosidase and α-amylase inhibitory activities of these compounds.
2. Results and Discussion
2.1. Isolation of Compounds
The methanol extract of the G. sylvestre leaves was suspended in water and then partitioned with n-hexane, CH2Cl2 and EtOAc to obtain four layers. The CH2Cl2 and water extracts were chromatographed using combined silica gel and RP-18 columns. The fractions were further purified by HPLC to give five new pregnane glycosides and four known compounds (Figure 1 and Supplementary Materials).
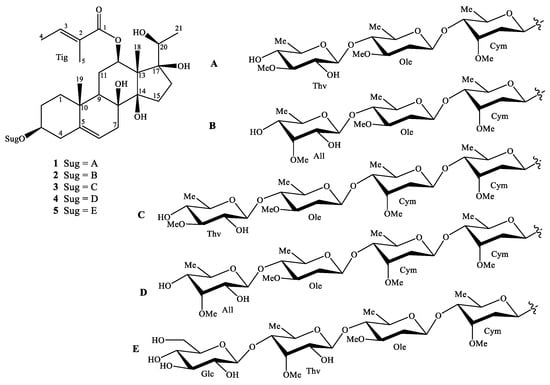
Figure 1.
Chemical structures of compounds of 1–5.
2.2. Compound Identification
Compound 1 was obtained as a white amorphous powder. Its molecular formula was determined as C47H76O17 by HRESIMS ion at m/z 935.4986 [M + Na]+ (calcd for [C47H76O17Na]+, 935.4975). The 1H-NMR spectrum showed proton signals of three methyl groups at δH 1.13 (3H, s), 1.53 (3H, s), and 1.05 (3H, d, J = 6.4 Hz), one olefinic proton at δH 5.32 (1H, br s), which represented a pregnane aglycone. Two methyl groups at δH 1.80 (3H, d, J = 7.2 Hz) and 1.85 (3H, s) and one olefinic proton at δH 6.98 (1H, q, J = 7.2 Hz), suggested the presence of a tigloyl moiety. Three anomeric protons [δH 4.84 (br d, J = 9.6 Hz), 4.57 (br d, J = 9.2 Hz), and 4.41 (br d, J = 8.0 Hz), three methoxy groups [δH 3.39, 3.42, and 3.60 (each 3H, s)], together with three secondary methyl groups [δH 1.19 (3H, d, J = 6.0 Hz), 1.25 (d, J = 6.0 Hz), and 1.35 (d, J = 6.0 Hz)], confirmed the presence of three sugar units. The 13C NMR and DEPT spectra indicated that 1 contained one carbonyl, seven non-protonated carbons (three oxygenated), nineteen methines (sixteen oxygenateds), nine methylenes, and eleven methyl carbons (three methoxys). The 1H and 13C-NMR spectroscopic data suggested that 1 was a pregnane glycoside [11]. Of these, 21 carbons were assigned to the pregnane skeleton, 5 to one tigloyl moiety, and 21 to a trisaccharide moiety. The HMBC correlations between H-19 (δH 1.13) and C-1 (δC 39.8)/C-5 (δC 140.0)/C-9 (δC 44.7)/C-10 (δC 38.0) suggested the position of a double bond at C-5/C-6. The HMBC correlations between H-18 (δH 1.53) and C-13 (δC 57.6)/C-14 (δC 89.3)/C-17 (δC 89.1); between H-21 (δH 1.05) and C-17 (δC 89.1)/C-20 (δC 71.5); and between H-6 (δH 5.32)/H-7 (δH 2.11)/H-9 (δH 1.49) and C-8 (δC 74.9) demonstrated the positions of hydroxyl groups at C-8, C-14, C-17, and C-20 (Figure 2). The constitution of the aglycone of 1 was demonstrated by the analysis of NOESY observations and similar reported-structures [11].

Figure 2.
The key HMBC, COSY, and NOESY correlations of compounds 1–5.
The aglycone of 1 was supposed to have the same configurations as those of gymnepregoside F and 12-O-(E)-cinnamoylgymnepregoside F from G. sylvestre [12], biogenetic derivatives of 1 at C-3, C-8, C-12, C-14, C-17, and C-20. In addition, the alkaline hydrolysis of 1 gave sarcostin, ((20S)-3β,8β,12β,14β,17β,20-hexahydroxypregn-5-ene) [13]. The multiplicity of H-12 [δH 4.68 (dd, J = 4.0, 11.6 Hz)] suggested that the configuration of H-12 was axial (α-configuration, Figure 2). The NOESY correlations between H-3 (δH 3.50) and Hα-1 (δH 1.09)/ Hα-4 (δH 2.33), and between H-12 (δH 4.68) and H-9 (δH 1.49)/Hα-15 (δH 1.88) suggested the configurations of the oxygenated groups at C-3 and C-12, the hydroxy groups at C-8 and C-14 to be β. The HMBC correlations between Tig H-5 (δH 1.85) and Tig C-1 (δC 169.1)/Tig C-2 (δC 130.0)/Tig C-3 (δC 139.5) and between Tig H-4 (δH 1.80) and Tig C-2/ Tig C-3 and NOESY correlations between Tig H-4 (δH 1.80) and Tig H-5 (δH 1.85) suggested the presence of (E)-tigloyl moiety. In addition, the position of this moiety at C-12 was confirmed by HMBC correlation from H-12 (δH 4.68) to Tig C-1 (δC 169.1). Acid hydrolysis of 1 gave three monosaccharides, which were identified as D-cymarose [14], D-oleandrose [14], and D-thevetose [15], by comparing their specific rotation with those reported [16]. The large coupling constants between H-1 and H-2 of monosaccharide moieties and also HMBC correlations between Thv H-1 (δH 4.41) and Ole C-4 (δC 84.1), Ole H-1 (δH 4.57) and Cym C-4 (δC 83.8), and between Cym H-1 (δH 4.84) and aglycone C-3 (δH 79.2) indicated the sugar linkages as β-D-thevetopyranosyl-(1→4)-β-D-oleandropyranosyl- (1→4)-β-D-cymaropyranoside and at C-3 of aglycone. Based on the above evidence, the structure of 1 was elucidated as (20S)-12β-tigloyloxy-3β,8β,14β,17β,20-pentahydroxypregn-5-ene 3-O-β-D-thevetopyranosyl- (1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranoside, a new compound named gymsyloside A.
The 1H and 13C-NMR spectra of 2 exhibited a pregnane aglycone, one tigloyl unit, and three sugar units (Table 1). In addition, the NMR data of 2 were similar to those of gymsyloside A (1), except for the difference of sugar unit at Ole C-4: D-thevetose replaced by 6-deoxy-3-O-methyl-D-allose. Acid hydrolysis of 2 confirmed the presence of D-cymarose, D-oleandrose, and 6-deoxy-3-O-methyl-D-allose as sugar components. Furthermore, the 1H and 13C-NMR data of 2 showed the sugar units as β-D-cymaropyranosyl, β-D-oleandropyranosyl, and 6-deoxy-3-O-methyl-β-D-allopyranose. The HMBC correlations between All H-1 (δH 4.70) and Ole C-4 (δC 84.0), Ole H-1 (δH 4.56) and Cym C-4 (δC 83.8), and between Cym H-1 (δH 4.85) and aglycone C-3 (δC 79.3) confirmed the sugar linkages to be 3-O-6-deoxy-3-O-methyl-β-D-allopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranoside. Consequently, compound 2 was elucidated to be (20S)-12β-tigloyloxy-3β,8β,14β,17β,20-pentahydroxypregn-5-ene 3-O-6-deoxy-3-O-methyl-β-D-allopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranoside, a new compound named gymsyloside B.

Table 1.
NMR spectroscopic data for compounds 1–3 in CD3OD.
The HRESIMS of 3 gave a pseudo-molecular ion peak at m/z 1079.5769 [M + Na]+, corresponding to the molecular formula of C54H88O20. The 1H and 13C-NMR spectra of 3 showed the presence of one pregnane aglycone, four sugar units, and one tigloyl unit (Table 1). The NMR data of 3 were compared to gymsyloside A (1) and found the addition of one sugar unit in the sugar linkages. Acid hydrolysis of 3 gave three monosaccharides, which were identified as D-cymarose, D-oleandrose, and D-thevetose. The tetrasaccharide was determined to be β-D-thevetopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside, by the analysis of HMBC and COSY correlations. The location of sugar linkages at C-3 was confirmed by the HMBC correlation between Cym I H-1 (δH 4.84) and C-3 (δC 79.3). Consequently, the structure of 3 was determined to be (20S)-12β-tigloyloxy-3β,8β,14β,17β, 20-pentahydroxypregn-5-ene 3-O-β-D-thevetopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside, a new compound named gymsyloside C.
The molecular formula of 4 was determined as C54H88O20 by the HRESIMS. The 1H and 13C-NMR data (Table 2) indicated that the structure of 4 was similar to those of 3, except for the difference of monosaccharide at Ole C-4. The sugar components were found to be similar to those of 2 (D-cymarose, D-oleandrose, and 6-deoxy-3-O-methyl-D-allose) [17]. Moreover, the sugar linkages, 3-O-6-deoxy-3-O-methyl-β-D-allopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside was confirmed by the HMBC correlations from All H-1 (δH 4.70) to Ole C-4 (δC 83.7), Ole H-1 (δH 4.56) to Cym II C-4 (δC 83.8), and from Cym II H-1 (δH 4.77) to Cym I C-4 (δC 83.8). Similar to those of 1–3, the aglycone was found as (20S)-12β-tigloyloxy-3β,8β,14β,17β, 20-pentahydroxypregn-5-ene. Consequently, the structure of 4 was determined as (20S)- 12β-tigloyloxy-3β,8β,14β,17β,20-pentahydroxypregn-5-ene 3-O-6-deoxy-3-O-methyl-β-D- allopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside and named gymsyloside D.

Table 2.
NMR spectroscopic data for compounds 4 and 5 in CD3OD.
The molecular formula of 5, C53H86O22 was determined by the HRESIMS pseudo-ion at m/z 1097.5530 [M + Na]+. The 1H and 13C NMR data of 5 were similar to 2, except for an additional sugar unit at Thv C-4 (Table 2). The sugar moieties were determined as D-cymarose [14], D-oleandrose [14], D-thevetose [15], and D-glucose [17] by acid hydrolysis. The HMBC correlations between Glc H-1 (δH 4.41) and Thv C-4 (δC 82.9), Thv H-1 (δH 4.43) and Ole C-4 (δC 84.1), Ole H-1 (δH 4.57) and Cym C-4 (δC 83.8), and between Cym H-1 (δH 4.84) and aglycone C-3 (δC 79.3) confirmed the sequence of sugar linkages, previously reproted from G. sylvestre [5]. Thus, compound 5 was characterized as (20S)-12β-tigloyloxy-3β,8β,14β,17β,20-pentahydroxypregn-5-ene 3-O-β-D-glucopyranosyl-(1→4)-β-D-thevetopyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranoside and named gymsyloside E.
The known compounds were identified as 3β-O-β-D-glucopyranosyl (1→6)-β-D-glucopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester (6) [7], gymnemoside-W1 (7) [18], 3β-O-β-D-xylopyranosyl-(1→6)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester (8) [7], and alternoside XIX (9) [18]. These compounds were already reported from G. sylvestre. Thus, for oleanane saponins, the main components could be hightly species-specific of G. sylvestre [7,18]. In addition, new pregnane glycosides were also found in G. alternifolium [19], G. sylvestre [5], and G. griffithii [15]. Five new pregnane glycosides in this report will contribute specific compounds in Gymnema genus.
2.3. α-Glucosidase and α-Amylase Inhibitory Activities
All compounds were evaluated for the α-glucosidase and α-amylase inhibitory assays. Compound 4 showed the weak α-glucosidase inhibitory activity (16.4 ± 2.3%) at the concentration of 200 µM, compared tos the positive control, acarbose (inhibition percentage of 57.8 ± 3.2% at the concentration of 155 µM) (Figure 3). Compounds 2–4 showed α-amylase inhibitory activity with inhibition percent ranging from 57.9% to 66.8% at the concentration of 200 µM (Figure 4). In the subsequent concentration-dependent assay, compounds 2, 3, and 4 showed significant α-amylase inhibitory activity, with IC50 values of 175.8 ± 2.3, 162.2 ± 2.7, and 113.0 ± 0.7 µM, respectively, compared to positive control, acarbose (IC50 value of 72.4 ±0.8 µM). This is the first report of α-glucosidase and α-amylase inhibitory activities of compounds from G. sylvestre. Recent reports have shown insulin secretion stimulation of G. sylvestre extract [4], antihyperglycemic effects of gmynemic acids [20], α-glucosidase and α-amylase inhibitory activities of pregnane glycosides from G. latifolium [11]. Previous studies have indicated that pregnane glycosides from G. griffithii showed moderate α-glucosidase inhibitory activity [15]. Russelioside B, a pregnane glycoside, possessed antidiabetic and antihyperlipidemic effect in streptozotocin induced diabetic rats [21]. Therefore, the results suggest that the discovery of pregane glycosides may increase the possibility of finding antidiabetic agents.
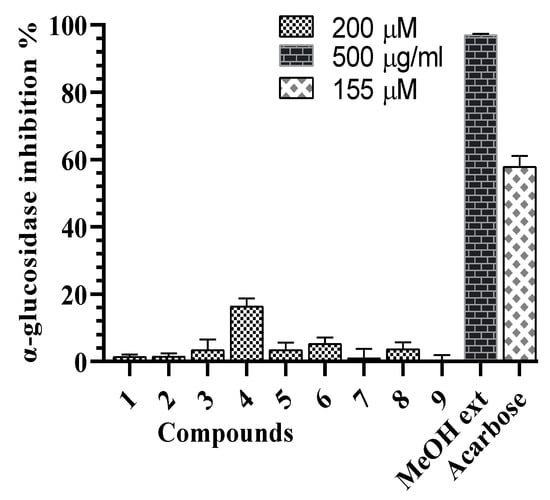
Figure 3.
α-Glucosidase inhibitory effects of the G. sylvestre extract and compounds 1–9.

Figure 4.
α-Amylase inhibitory effects of the G. sylvestre extract and compounds 1–9.
3. Materials and Methods
3.1. General
All NMR spectra were recorded on an Agilent 400-MR-NMR (Agilent technologies, Santa Clara, CA, USA) spectrometer operated at 400 and 100 MHz for hydrogen and carbon, respectively. Data processing was carried out with the MestReNova ver.6.0.2 program. HRESIMS spectra were obtained using an AGILENT 6550 iFunnel Q-TOF LC/MS system (Agilent technologies, Santa Clara, CA, USA). Optical rotations were determined on a Jasco DIP-370 automatic polarimeter. Preparative HPLC was carried out using an AGILENT 1200 HPLC system. Column chromatography was performed on silica-gel (Kieselgel 60, 70–230 mesh and 230–400 mesh, Merck) or YMC RP-18 resins (30–50 µm, Fuji Silysia Chemical Ltd., Aichi, Japan). For thin layer chromatography (TLC), a pre-coated silica-gel 60 F254 (0.25 mm, Merck, Darmstadt, Germany) and RP-18 F254S plates (0.25 mm, Merck, Darmstadt, Germany) were used.
3.2. Plant Material
The leaves of Gymnema sylvestre (Retz.) R.Br. ex Sm. were collected in Hai Loc, Hai Hau, Nam Dinh in November, 2015, and identified by Dr. Nguyen The Cuong, Institute of Ecology and Biological Resources. A voucher specimen (NCCT-P20) was deposited at the Herbarium Institute of Marine Biochemistry, VAST.
3.3. Extraction and Isolation
The dried powders of G. sylvestre leaves (4.0 kg) were sonicated with hot methanol (3 times × 10 L, each 3 h) to give MeOH extract (450 g), after evaporation of the solvent. The MeOH extract was suspended in water and successively partitioned with n-hexane, CH2Cl2 and EtOAc to obtain the n-hexane (GS1, 47.0 g), CH2Cl2 (GS2, 60.0 g), EtOAc extracts (GS3, 27.0 g) and H2O layer (GS4). GS2 was chromatographed on a silica gel column (180.0 g, silica gel) eluting with gradient solvent of n-hexane:acetone (40:1, 20:1, 10:1, 5:1, 1:1, and 0:1, v/v, each 2 L), to give seven fractions, GS2A-GS2G. The GS2F fraction was chromatographed on a silica gel column eluting with CHCl3:MeOH (11:1, v/v) to give four fractions, GS2F1-GS2F4. GS2F1 was chromatographed on a RP-18 column using MeOH:H2O (4:1, v/v) as a solvent, to give five fractions, GS2F1A-GS2F1E. Compounds 1 (17.0 mg, tR 38.5 min) and 2 (9.0 mg, tR 42.1 min) were yielded from GS2F1B fraction using HPLC system: J’sphere H-80 column (150 × 20 mm), flow rate of 3 mL/min, and solvent condition of 40% acetonitrile in water. Compounds 3 (5.0 mg, tR 44.7 min) and 4 (5.0 mg, tR 49.4 min) were yielded from GS2F1D fraction on J’sphere H-80 column (150 × 20 mm), flow rate of 3 mL/min, and solvent condition of 40% acetonitrile in water. GS2G was chromatographed on RP-18 column eluting with MeOH:H2O (4:1, v/v) to give four smaller fractions, GS2G1-GS2G4. Finally, GS2G3 was chromatographed on J’sphere H-80 column (150 × 20 mm), flow rate of 3 mL/min, and solvent condition of 35% acetonitrile in water to yield compound 5 (14.0 mg, tR 39.7 min). GS4 was chromatographed on a Diaion column and eluted with H2O then increased concentrations of MeOH in H2O, to obtain sub-fractions, GS4A-GS4C. GS4C was chromatographed on a silica gel column eluting with a gradient of CHCl3:MeOH (20:1, 10:1, 5:1, 1:1, v/v) to give smaller fractions, GS4C1-GS4C4. GS4C4 was chromatographed on an RP-18 CC eluting with MeOH:water (2:1, v/v) to give smaller fractions, GS4C4A-GS4C4E. GS4C4B was chromatographed on an RP-18 column eluting with acetone:H2O (0.8:1, v/v) to yield 7 (5.0 g) and 9 (40.0 mg). GS4C4E was chromatographed on an RP-18 column eluting with acetone:H2O (1:1, v/v), to yield 6 (100.0 mg) and 8 (5.0 mg).
3.3.1. Gymsyloside A (1)
White amorphous powder; −20.0 (c 0.1, MeOH); C47H76O17, HRESIMS m/z: 935.4986 [M + Na]+ (calcd for [C47H76O17Na]+, 935.4975); 1H (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data, see Table 1.
3.3.2. Gymsyloside B (2)
White amorphous powder; + 35.0 (c 0.1, MeOH); C47H76O17, HRESIMS m/z: 935.4996 [M + Na]+ (calcd for [C47H76O17Na]+, 935.4975); 1H (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data, see Table 1.
3.3.3. Gymsyloside C (3)
White amorphous powder; + 80.0 (c 0.1, MeOH); C54H88O20, HRESIMS m/z: 1079.5769 [M + Na]+ (calcd for [C54H88O20Na]+, 1079.5769); 1H (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data, see Table 1.
3.3.4. Gymsyloside D (4)
White amorphous powder; + 58.7 (c 0.1, MeOH); C54H88O20, HRESIMS m/z: 1079.5778 [M + Na]+ (calcd for [C54H88O20Na]+, 1079.5769); 1H (CD3OD, 400 MHz) and 13C-NMR (CD3OD, 100 MHz) data, see Table 2.
3.3.5. Gymsyloside E (5)
White, amorphous powder; + 54.0 (c 0.1, MeOH); C53H86O22, HRESIMS m/z: 1097.5530 [M + Na]+ (calcd for [C53H86O22Na]+, 1097.5503); 1H (CD3OD, 400 MHz) and 13C NMR (CD3OD, 100 MHz) data, see Table 2.
3.4. Acid Hydrolysis
Each compound (1–5, 3.0 mg) was separately dissolved in 1.0 N HCl (dioxane—H2O, 1:1, v/v, 1.0 mL) and heated to 80 °C in a water bath for 3 h. The acidic solution was dried under N2 overnight. After extraction with CHCl3, the aqueous layer was dried using N2 to give aqueous residue (A). The aqueous residue (A) was separated by silica gel CC eluting with CH2Cl2–MeOH (10:1, v/v) and then further fractionated by RP-18 CC using a solvent gradient of MeOH–H2O (6:4, 7:3, and 8:2, v/v), to give the monosaccharides (50% yield). The specific rotations of these sugars were determined. The specific rotations () of sugars was determined after dissolving in H2O for 24 h and compared to the literature (lit):D-cymarose: found +50.1 (c 0.4, H2O), lit +51.8 [14]; D-oleandrose: found −12.1 (c 0.4, H2O), lit +11.7 [14]; D-thevetose: found +40.3 (c 0.4, H2O); lit +42.3 [15]; 6-deoxy-3-O-methyl-D-allose: found +10.9 (c 0.4, H2O); lit +10.0 [17]; D-glucose: found + 49.2 (c 0.4, H2O); lit +48.0 [17]. Based on the above evident, sugar components were found in: compounds 1 and 3: D-cymarose, D-oleandrose, and D-thevetose; compounds 2 and 4: D-cymarose, D-oleandrose, and 6-deoxy-3-O-methyl-D-allose; compound 5: D-cymarose, D-oleandrose, D-thevetose, and D-glucose.
3.5. Alkaline Hydrolysis
A solution of compound 1 (8 mg) in 1.0 mL of 5% KOH/MeOH was heated at 40 °C four 4 h and then neutralized with HCl 0.1 M. After that, the solution was partitioned with CHCl3 to give CHCl3 layer. CHCl3 layer was separated on HPLC system: J’sphere H-80 column (150 × 20 mm), solvent condition of 55% acetonitrile, to give sarcostin (54% yield). In a similar way, sarcostin was found as aglycone of compounds 2–5.
3.6. α-Glucosidase Inhibitory Assay
The α-glucosidase (G0660-750UN, Sigma-Aldrich, St. Louis, MO) enzyme inhibition assay was performed according to the previously described method [11]. The sample solution (2 mL dissolved in dimethyl sulfoxide (DMSO)) and 0.5 U/mL α-glucosidase (40 mL) were mixed in 120 mL of 0.1 M phosphate buffer (pH 7.0). After 5 min pre-incubation, 5 mM p-nitrophenyl-α-D-glucopyranoside solution (40 mL) was added, and the solution was incubated at 37 °C for 30 min. The absorbance of released 4-nitrophenol was measured at 405 nm by using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
3.7. α-Amylase Inhibitory Assay
The α-amylase (A8220, Sigma-Aldrich, St. Louis, MO, USA) enzyme inhibitory activity was measured using the reported method [11]. Substrate was prepared by boiling 100 mg potato starch in 5 mL phosphate buffer (pH 7.0) for 5 min, then cooling to room temperature. The samples (2 mL dissolved in DMSO) and substrate (50 mL) were mixed in 30 mL of 0.1 M phosphate buffer (pH 7.0). After 5 min pre-incubation, 5 mg/mL α-amylase solution (20 mL) was added, and the solution was incubated at 37 °C for 15 min. The reaction was stopped by adding 50 mL 1 M HCl and then 50 mL iodine solution was added. The absorbances were measured at 650 nm by a microplate reader.
4. Conclusions
In summary, five new pregnane glycosides and four known oleanane saponins were isolated and identified from G. sylvestre. Besides oleanane saponins-hightly species-specific of G. sylvestre, new pregnane glycosides in this report will provide secondary metabolisms as specific compounds in Gymnema genus. Compound 4 showed the weak α-glucosidase inhibitory activity. Compounds 2, 3, and 4 showed significant α-amylase inhibitory activity. This is the first report of α-amylase and α-glycosidase inhibitory activities of compounds from G. sylvestre.
Supplementary Materials
Figure S1: The chemical structures of compounds 6–9; Figure S2: HR-ESI-MS of compound 1, Figure S3: 1H-NMR spectrum of compound 1, Figure S4: 13C-NMR spectrum of compound 1, Figure S5: DEPT135 spectrum of compound 1, Figure S6: HSQC spectrum of compound 1, Figure S7: HMBC spectrum of compound 1, Figure S8: COSY spectrum of compound 1, Figure S9: ROESY spectrum of compound 1, Figure S10: HR-ESI-MS of compound 2, Figure S11: 1H-NMR spectrum of compound 2, Figure S12: 13C-NMR spectrum of compound 2, Figure S13: DEPT135 spectrum of compound 2, Figure S14: HSQC spectrum of compound 2, Figure S15: HMBC spectrum of compound 2, Figure S16: COSY spectrum of compound 2, Figure S17: ROESY spectrum of compound 2, Figure S18: HR-ESI-MS of compound 3, Figure S19: 1H-NMR spectrum of compound 3, Figure S20: 13C-NMR spectrum of compound 3, Figure S21: HSQC spectrum of compound 3, Figure S22: HMBC spectrum of compound 3, Figure S23: COSY spectrum of compound 3, Figure S24: ROESY spectrum of compound 3, Figure S25: HR-ESI-MS of compound 4, Figure S26: 1H-NMR spectrum of compound 4, Figure S27: 13C-NMR spectrum of compound 4, Figure S28: DEPT135 spectrum of compound 4, Figure S29: HSQC spectrum of compound 4, Figure S30: HMBC spectrum of compound 4, Figure S31: COSY spectrum of compound 4, Figure S32: ROESY spectrum of compound 4, Figure S33: HR-ESI-MS of compound 5, Figure S34: 1H-NMR spectrum of compound 5, Figure S35: 13C-NMR spectrum of compound 5, Figure S36: DEPT135 spectrum of compound 5, Figure S37: HSQC spectrum of compound 5; Figure S38: HMBC spectrum of compound 5; Figure S39: COSY spectrum of compound 5; Figure S40: 1H-NMR spectrum of compound 6; Figure S41: 13C-NMR spectrum of compound 6; Figure S42: HSQC spectrum of compound 6; Figure S43: 1H-NMR spectrum of compound 7; Figure S44: 13C-NMR spectrum of compound 7; Figure S45: HSQC spectrum of compound 7; Figure S46: 1H-NMR spectrum of compound 8; Figure S47: 13C-NMR spectrum of compound 8; Figure S48: HSQC spectrum of compound 8; Figure S49: 1H-NMR spectrum of compound 9; Figure S50: 13C-NMR spectrum of compound 9; Figure S51: HSQC spectrum of compound 9.
Author Contributions
Isolation, P.V.K., D.T.H.Y., and B.H.T.; Formal analysis, P.V.K., D.T.H.Y., N.X.N., B.H.T., D.T.T., P.H.Y., T.M.N., J.H.L., S.Y.K. and S.H.K.; Research idea, N.V.H., T.M.N., and C.V.M.; Structure elucidation, P.V.K., D.T.H.Y., N.X.N., and B.H.T.; Biological assay, D.T.H.Y., S.P., J.H.L., S.Y.K., and S.H.K.; Writing, P.V.K., D.T.H.Y., N.X.N., N.V.H., and S.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED), under grant number 104.01-2017.25 and by the National Research Foundation of Korea (NRF) grant [NRF-2016R1A2B4006742], funded by the Ministry of Education, Science and Technology, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thangavelu, D.; Thangavelu, T.; Vembu, T.; Venkatachalam, K.; Selladurai, E.; Jegadeesan, M. Pharmacognostic and phytochemical studies on Gymnema sylvestre R. Br. hairy variant. Int. J. Pharm. Phytopharm. Res. 2012, 2, 143–147. [Google Scholar]
- Bala Krishna, M. Review on: Gymnema sylvestre. J. Pharm. Res. 2011, 4, 3963–3965. [Google Scholar]
- Ahmed, A.B.A.; Rao, A.S.; Rao, M.V. Role of in vivo leaf and in vitro callus of Gymnema sylvestre in maintaining the normal levels of blood glucose and lipid profile in diabetic Wistar rats. Biomedicine 2008, 28, 134–138. [Google Scholar]
- Al-Romaiyan, A.; Liu, B.; Asare-Anane, H.; Maity, C.R.; Chatterjee, S.K.; Koley, N.; Biswas, T.; Chatterji, A.K.; Huang, G.C.; Amiel, S.A.; et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother. Res. 2010, 24, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Y.; Zhang, Y.; Ren, F.; Xu, J.; Yu, N.; Zhao, Y. New pregnane glycosides from Gymnema sylvestre. Molecules 2015, 20, 3050–3066. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.P.; Mahato, S.B.; Sarkar, S.K.; Poddar, G. Triterpenoid saponins from Gymnema sylvestre. Phytochemistry 1996, 41, 1181–1185. [Google Scholar] [CrossRef]
- Ye, W.-C.; Zhang, Q.-W.; Liu, X.; Che, C.-T.; Zhao, S.-X. Oleanane saponins from Gymnema sylvestre. Phytochemistry 2000, 53, 893–899. [Google Scholar] [CrossRef]
- Vats, S.; Kamal, R. Identification of flavonoids from plant parts and callus culture of Gymnema sylvestre R.Br.: An antidiabetic plant. Curr. Bioact. Compd. 2016, 12, 264–268. [Google Scholar] [CrossRef]
- Trang, D.T.; Yen, D.T.H.; Cuong, N.T.; Anh, L.T.; Hoai, N.T.; Tai, B.H.; Doan, V.V.; Yen, P.H.; Quang, T.H.; Nhiem, N.X.; et al. Pregnane glycosides from Gymnema inodorum and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2019, 1–7, in press. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Kiem, P.V.; Minh, C.V.; Ban, N.K.; Cuong, N.X.; Tung, N.H.; Minh, H.L.; Ha, D.T.; Tai, B.H.; Quang, T.H.; et al. α-Glucosidase inhibition properties of cucurbitane-type triterpene glycosides from the fruits of Momordica charantia. Chem. Pharm. Bull. 2010, 58, 720–724. [Google Scholar] [CrossRef]
- Yen, D.T.H.; Trang, D.T.; Tai, B.H.; Doan, V.V.; Yen, P.H.; Nhiem, N.X.; Van Minh, C.; Hoang Duc, M.; Park, S.; Hyuk Lee, J.; et al. Four new pregnane glycosides from Gymnema latifolium and their α-glucosidase and α-amylase inhibitory activities. Nat. Prod. Res. 2020, 1–8, in press. [Google Scholar] [CrossRef] [PubMed]
- Yen, D.T.H.; Thu, V.K.; Tai, B.H.; Yen, P.H.; Nhiem, N.X.; Kiem, P.V. Pregnane glycosides from Gymnema sylvestre. Vietnam, J. Chem. 2019, 57, 208–212. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Ye, Y.; Chen, F.; Tu, J.; Pan, Y. Three new immunomodulating C21-steroidal glycosides from the stems of Stephanotis mucronata. Chem. Biodivers 2005, 2, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Warashina, T.; Noro, T. Steroidal glycosides from the aerial part of Asclepias incarnata. Phytochemistry 2000, 53, 485–498. [Google Scholar] [CrossRef]
- Srisurichan, S.; Puthong, S.; Pornpakakul, S. Pregnane-type steroidal glycosides from Gymnema griffithii Craib. Phytochemistry 2014, 106, 197–206. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T. Pregnane glycosides from the roots of Asclepias tuberosa. Chem. Pharm. Bull. 2000, 48, 1017–1022. [Google Scholar] [CrossRef]
- Abe, F.; Okabe, H.; Yamauchi, T.; Honda, K.; Hayashi, N. Pregnane glycosides from Marsdenia tomentosa. Chem. Pharm. Bull. 1999, 47, 869–875. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Xie, P.; Di, Y.-T.; Peng, S.-L.; Ding, L.-S.; Wang, M.-K. Two new triterpenoid saponins from Gymnema sylvestre. J. Integr. Plant Biol. 2008, 50, 589–592. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Matsuchika, K.; Arihara, S.; Chang, H.-C.; Wang, J.-D. Pregnane glycosides, gymnepregosides A-F from the roots of Gymnema alternifolium. Chem. Pharm. Bull. 1998, 46, 1239–1243. [Google Scholar] [CrossRef]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; El-Maraghy, S.A.; El-Dine, R.S.; Rizk, S.M. Russelioside B, a pregnane glycoside ameliorates hyperglycemia in streptozotocin induced diabetic rats by regulating key enzymes of glucose metabolism. Chemico-Biol. Interact. 2016, 252, 47–53. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).