Antiplasmodial Activity of Nitroaromatic Compounds: Correlation with Their Reduction Potential and Inhibitory Action on Plasmodium falciparum Glutathione Reductase
Abstract
:1. Introduction
2. Results
2.1. Relationship between Antiplasmodial Activity of Nitroaromatic Compounds and Their Single-Electron Reduction Midpoint Potential
2.2. Single-Electron Reduction of Nitroaromatics by PfFNR and PfGR
2.3. Inhibition of P. falciparum Glutathione Reductase by Nitroaromatic Compounds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Enzyme Kinetic Studies
4.2.2. Antiplasmodial In Vitro Activity Studies
4.2.3. Statistical Analysis and Calculations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ArNO2 | Nitroaromatic compound |
| E17 | Single-electron reduction midpoint potential of nitroaromatic compound (redox potential of ArNO2/ArNO2−. couple) at pH 7.0 |
| HGR | Human erythrocyte glutathione reductase |
| IC50 | Compound concentration causing 50% parasite growth inhibition |
| kcat | Enzyme catalytic constant |
| kcat/Km | Enzyme bimolecular rate constant (catalytic efficiency) |
| Ki | Enzyme inhibition constant |
| log D | Octanol/water distribution coefficient at pH 7.0 |
| PfFNR | P. falciparum ferredoxin:NADP+ oxidoreductase |
| PfGR | P. falciparum glutathione reductase |
| ROS | Reactive oxygen species |
| TNT | 2,4,6-Trinitrotoluene |
| TR | Trypanothione reductase |
References
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisancia, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa betwen 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol. Microbiol. 2004, 53, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Pelfrene, E.; Harvey Allchurch, M.; Ntamabyaliro, N.; Nambasa, A.; Ventura, F.V.; Nagercoil, N.; Cavaleri, M. The European Medicines Agency’s scientific opinion on oral feximidazole for human African trypanosomiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007381. [Google Scholar] [CrossRef]
- Guissani, A.; Henry, Y.; Lougmani, N.; Hickel, B. Kinetic studies of four types of nitroheterocyclic radicals by pulse radiolysis. Correlation of pharmacological properties to decay rates. Free Radic. Biol. Med. 1990, 8, 173–189. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Bot, C.; Kelly, J.M.; Hall, B.S. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr. Top. Med. Chem. 2011, 11, 2072–2084. [Google Scholar] [CrossRef]
- Pal, C.; Bandyopadhyay, H. Redox active antiparasitic drugs. Antiox. Redox Signal. 2012, 17, 555–582. [Google Scholar] [CrossRef]
- Bironaitė, D.A.; Čėnas, N.K.; Kulys, J.J. The rotenone-insensitive reduction of quinones and nitrocompounds by mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta 1991, 1060, 203–209. [Google Scholar] [CrossRef]
- Čėnas, N.; Nemeikaitė-Čėnienė, A.; Sergedienė, E.; Nivinskas, H.; Anusevičius, Ž.; Šarlauskas, J. Quantitative structure-activity relationships in enzymatic single-electron reduction of nitroaromatic explosives: Implications for their cytotoxicity. Biochim. Biophys. Acta 2001, 1528, 31–38. [Google Scholar] [CrossRef]
- Anusevičius, Ž.; Nivinskas, H.; Šarlauskas, J.; Sari, M.-A.; Boucher, J.-L.; Čėnas, N. Single-electron reduction of quinone and nitroaromatic xenobiotics by recombinant rat neuronal nitric oxide synthase. Acta Biochim. Pol. 2013, 60, 217–222. [Google Scholar] [CrossRef]
- Moussaoui, M.; Misevičienė, L.; Anusevičius, Ž.; Marozienė, A.; Lederer, F.; Baciou, L.; Čėnas, N. Quinones and nitroaromatic compounds as subversive substrates of Staphylococcus aureus flavohemoglobin. Free Rad. Biol. Med. 2018, 123, 107–115. [Google Scholar] [CrossRef]
- Henderson, G.B.; Ulrich, P.; Fairlamb, A.H.; Rosenberg, I.; Pereira, M.; Sela, I.; Cerami, A. “Subversive” substrates for the enzyme trypanothione disulfide reductase: Alternative approach to chemotherapy of Chagas disease. Proc. Natl. Acad. Sci. USA 1988, 85, 5374–5378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čėnas, N.; Bironaitė, D.; Dičkancaitė, E.; Anusevičius, Ž.; Šarlauskas, J.; Blanchard, J.S. Chinifur, a selective inhibitor and subversive substrate for Trypanosoma congolense trypanothione reductase. Biochem. Biophys. Res. Commun. 1994, 204, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Millet, R.; Maes, L.; Landry, V.; Sergheraert, C.; Davioud-Charvet, E. Antitrypanosomal activities and cytotoxicity of 5-nitro-2-furancarbohydrazides. Bioorg. Med. Chem. Lett. 2002, 12, 3601–3604. [Google Scholar] [CrossRef]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.; Prieto, A.A.; Iglesias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1089–1097. [Google Scholar] [CrossRef]
- Leroux, A.E.; Krauth-Siegel, R.L. Thiol redox biology of trypanosomatids as potential targets for chemotherapy. Mol. Biochem. Parasitol. 2016, 206, 67–74. [Google Scholar] [CrossRef]
- Ilari, A.; Genovese, I.; Fiorillo, F.; Battista, T.; De Iona, I.; Fiorello, A.; Colotti, G. Toward a drug against all kinetoplastids: From LeishBox to specific and potent trypanothione reductase inhibitors. Mol. Pharm. 2018, 15, 3069–3078. [Google Scholar] [CrossRef]
- Kuntz, A.N.; Davioud-Charvet, E.; Dessolin, J.; Sayed, A.A.; Califf, L.L.; Arnér, E.S.J.; Williams, D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: An essential parasite enzyme and a key drug target. PLoS Med. 2007, 4, e264. [Google Scholar]
- Li, T.; Ziniel, P.D.; He, P.Q.; Kommer, V.P.; Crowther, G.J.; He, M.; Lin, Q.; Van Voorhis, W.C.; Williams, D.L.; Wang, M.W. High-throughput screening against thioredoxin glutathione reductase identifies novel inhibitors with potential therapeutic value for schistosomiasis. Infect. Dis. Poverty 2015, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Grellier, P.; Šarlauskas, J.; Anusevičius, Ž.; Marozienė, A.; Houeee-Levin, C.; Schrevel, J.; Čėnas, N. Antiplasmodial activity of nitroaromatic and quinoidal compounds: Redox potential vs inhibition of erythrocyte glutathione reductase. Arch. Biochem. Biophys. 2001, 393, 199–206. [Google Scholar] [CrossRef]
- Wiesner, J.; Kettler, K.; Sakowski, J.; Ortmann, R.; Jomaa, H.; Schlitzer, M. Structure-activity relationships of novel anti-malarial agents: Part 5. N-(4-acylamino-3-benzoylphenyl)-[5-(4-nitrophenyl)-2-furyl]acrylic acid amides. Bioorg. Med. Chem. Lett. 2003, 13, 361–363. [Google Scholar] [CrossRef]
- Tukulula, M.; Sharma, R.-K.; Meurillon, M.; Mahajan, A.; Naran, K.; Warner, D.; Huang, J.; Mekonnen, B.; Chibale, K. Synthesis and antiplasmodial and antimycobacterial evaluation of new nitroimidazole and nitroimidazooxazine derivatives. ACS Med. Chem. Lett. 2012, 4, 128–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munigunti, R.; Gathiaka, S.; Acevedo, O.; Sahu, R.; Tekwari, B.; Calderon, A.I. Characterization of PfTrxR inhibitors using antimalarial assays and in silico techniques. Chem. Cent. J. 2013, 7, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkard, L.; Scheuermann, A.; Simithy, J.; Calderon, A.I. Development of a functional assay to detect inhibitors of Plasmodium falciparum glutathione reductase utilizing liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2015, 30, 543–547. [Google Scholar] [CrossRef]
- Vennerstrom, L.J.; Eaton, J.W. Oxidants, oxidant drugs, and malaria. J. Med. Chem. 1988, 31, 1269–1277. [Google Scholar] [CrossRef]
- Čėnas, N.K.; Bironaitė, D.A.; Kulys, J.J.; Sukhova, N.M. Interaction of nitrofurans with glutathione reductase. Biochim. Biophys. Acta 1991, 1073, 195–199. [Google Scholar]
- Cakmak, R.; Durdagi, S.; Ekinci, D.; Sentürk, M.; Topal, G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5398–5402. [Google Scholar] [CrossRef]
- Sarma, G.N.; Savvides, S.N.; Becker, K.; Schirmer, M.; Schirmer, R.H.; Karplus, P.A. Glutathione reductase of the malarial parasite Plasmodium falciparum: Crystal structure and inhibitor development. J. Mol. Biol. 2003, 328, 893–907. [Google Scholar] [CrossRef]
- Tyagi, C.; Bathke, J.; Goyal, S.; Fischer, M.; Dahse, H.M.; Chacko, S.; Becker, K.; Grover, A. Targeting the intersubunit cavity of Plasmodium falciparum glutathione reductase by a novel natural inhibitor: Computational and experimental evidence. Int. J. Biochem. Cell Biol. 2015, 61, 72–80. [Google Scholar] [CrossRef]
- Böhme, C.C.; Arscott, D.; Becker, K.; Schirmer, R.H.; Williams, C.H., Jr. Kinetic characterization of glutathione reductase from the malarial parasite Plasmodium falciparum. Comparison with the human enzyme. J. Biol. Chem. 2000, 275, 37317–37323. [Google Scholar] [CrossRef] [Green Version]
- Grellier, P.; Marozienė, A.; Nivinskas, H.; Šarlauskas, J.; Aliverti, A.; Čėnas, N. Antiplasmodial activity of quinones: Roles of aziridinyl substituents and the inhibition of Plasmodium falciparum glutathione reductase. Arch. Biochem. Biophys. 2010, 494, 32–39. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Wong, W.C.; Silva, J.; Khan, S. Toxicity of nitrobenzene compounds towards isolated hepatocytes: Dependence on reduction potential. Xenobiotica 1990, 20, 945–955. [Google Scholar] [CrossRef]
- Lukevits, E.; Demicheva, L. Biological activity of furan derivatives. Chem. Heterocycl. Comp. (Riga) 1993, 3, 242–266. [Google Scholar]
- Daghastanli, N.A.; Degterev, L.A.; Tedesco, A.C.; Borisevitch, I.E. Phototoxicity of a 5-nitrofuran-ethenyl-quinoline antiseptic (Quinifuryl) to P388 mouse leukemia cells. Braz. J. Med. Biol. Res. 2004, 37, 1873–1879. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.H.; Tzeng, C.C.; Chiu, C.C.; Hsu, C.Y.; Chou, C.-K.; Chen, Y.L. Discovery of 2-[2-(5-nitrofuran-2-yl) vinyl]quinoline derivatives as a novel type of antimetastatic agents. Bioorg. Med. Chem. 2015, 23, 141–148. [Google Scholar] [CrossRef]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Balconi, E.; Pennati, A.; Crobu, D.; Pandini, V.; Cerutti, R.; Zanetti, G.; Aliverti, A. The ferreroxin-NADP+ reductase/ferredoxin electron transfer system of Plasmodium falciparum. FEBS J. 2009, 276, 4249–4260. [Google Scholar] [CrossRef]
- Seeber, F.; Aliverti, A.; Zanetti, G. The plant-type ferredoxin-NADP+ reductase/ferredoxin redox system as a possible drug target against apicomplexan human parasites. Current Pharm. Des. 2005, 11, 3159–3172. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the essential genes of the human parasite Plasmodium falciparum by saturation mutagenesis. Science 2018, 360, eaap7847. [Google Scholar] [CrossRef] [Green Version]
- Bironaite, D.A.; Chenas, N.K.; Kulis, Y.Y. Nonphysiological redox agents are reduced in the NADP(H) binding-site of glutathione reductase. Biochemistry-Moscow 1992, 57, 818–820. (in Russian). [Google Scholar]
- Belorgey, D.; Lanfranchi, D.A.; Davioud-Charvet, E. 1,4-Naphthoquinones and other NADPH- dependent glutathione reductase-catalyzed redox cyclers as antimalarial agents. Curr. Pharm. Des. 2013, 10, 2512–2528. [Google Scholar] [CrossRef]
- Salmon-Chemin, L.; Lemaire, A.; De Freitas, S.; Deprez, B.; Sergheraert, C.E.; Davioud-Charvet, E. Parallel synthesis of a library of 1,4-naphthoquinones and automatic screening of potential inhibitors of trypanothione reductase from Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2000, 10, 631–635. [Google Scholar] [CrossRef]
- Miškinienė, V.; Anusevičius, Ž.; Marozienė, A.; Kliukienė, R.; Nivinskas, H.; Šarlauskas, J.; Čėnas, N.; Becker, K. Tetryl as inhibitor and subversive substrate for human erythrocyte glutathione reductase. In Proceedings of the 13th International Symposium on Flavins and Flavoproteins, Konstanz, Germany, 31 August–4 September 1999; Ghisla, S., Kroneck, P., Macheroux, P., Sund, H., Eds.; Rudolph Weber: Berlin, Germany, 1999; pp. 703–706. [Google Scholar]
- Foley, M.; Tilley, L. Quinoline antimalarials: Mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar] [CrossRef]
- Famin, O.; Krugliak, M.; Ginsburg, H. Kinetics of inhibition of glutathione-mediated degradation of protoporphyrin IX by antimalarial drugs. Biochem. Pharmacol. 1999, 58, 59–68. [Google Scholar] [CrossRef]
- Marva, E.; Chevion, M.; Golenser, J. The effects of free radicals induced by paraquat and copper on the in vitro development of Plasmodium falciparum. Free Rad. Res. Comun. 1991, 12, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Sharma, S.; Patankar, S. Glutathione and thioredoxin systems of the malaria parasite Plasmodium falciparum: Partners in crime? Biochem. Biophys. Res. Commun. 2017, 488, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ayi, K.; Cappadoro, M.; Branca, M.; Turrini, F.; Arese, P. Plasmodium falciparum glutathione metabolism and growth are independent of glutathione system of host erythrocyte. FEBS Lett. 1998, 424, 257–261. [Google Scholar] [CrossRef] [Green Version]
- González-Párraga, P.; Hernández, A.; Argüelles, J.C. Role of antioxidant enzymatic defences against oxidative stress, H2O2, and the acquisition of oxidative tolerance in Candida albicans. Yeast 2003, 20, 1161–1169. [Google Scholar] [CrossRef]
- Dixit, V.; Pandey, V.; Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, R.C.; De Bem, A.F.; Locatelli, C.; Pedrosa, R.C.; Geremias, R.; Wilhelm Filho, D. Time-dependent oxidative stress caused by benznidazole. Redox Rep. 2001, 6, 265–270. [Google Scholar] [CrossRef]
- Čėnas, N.; Prast, S.; Nivinskas, H.; Šarlauskas, J.; Arner, E.S.J. Interaction of nitroaromatic compounds with the mammalian selenoprotein thioredoxin reductase and the relation to induction of apoptosis in human cancer cells. J. Biol. Chem. 2006, 281, 5593–5603. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.; Basilico, N.; Parapini, S.; Pasini, E.; Olliaro, P.; Taramelli, D. Does chloroquine really act through oxidative stress? FEBS Lett. 2002, 522, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Besset, T.; Moutet, J.-C.; Fayolle, M.; Brückner, M.; Limosin, D.; Becker, K.; Davioud-Charvet, E. The aza-analogues of 1,4-naphthoquinones are potent substrates and inhibitors of plasmodial thioredoxin and glutathione reductases and of human erythrocyte glutathione reductase. Org. Biomol. Chem. 2008, 6, 2731–2742. [Google Scholar] [CrossRef]
- Dong, C.K.; Patel, V.; Yang, J.C.; Dvorin, J.D.; Duraisingh, M.T.; Clardy, J.; Wirth, D.F. Type II NADH dehydrogenase of the respiratory chain of Plasmodium falciparum and its inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 972–975. [Google Scholar] [CrossRef] [Green Version]
- Čėnas, N.K.; Arscott, D.; Williams, C.H., Jr.; Blanchard, J.S. Mechanism of reduction of quinones by Trypanosoma congolense trypanothione reductase. Biochemistry 1994, 33, 2509–2515. [Google Scholar] [CrossRef]
- Kröger, M.; Fels, G. 14C-TNT synthesis revisited. J. Label. Compd. Radiopharm. 2000, 43, 217–227. [Google Scholar] [CrossRef]
- Hughes, E.D.; Ingold, C.; Pearson, R.B. Nitration at nitrogen and oxygen centers. Part I. Kinetics and conversion of secondary amines into nitroamines. J. Chem. Soc. 1958, 4357–4365. [Google Scholar] [CrossRef]
- Khan, A.H.; Ross, W.C.J. Tumor-growth inhibitory nitrophenylaziridines and related compounds: Structure-activity relationships. Chem. Biol. Interact. 1969, 1, 27–47. [Google Scholar] [CrossRef]
- Sukhova, N.M.; Lidaka, M.J.; Voronova, V.A.; Zidermane, A.A.; Kravchenko, I.M.; Dauvarte, A.Z.; Preisa, I.E.; Meirena, I.E. Substituted 2-[2′-(5”-nitrofuryl-2”)vinyl- and 4′-(5”-nitrofuryl-2”)-1,3- butadienyl)-quinoline-4-carboxylic acid amides and salts thereof. U.S. Patent 4201784A, 6 May 1980. [Google Scholar]
- Lukevits, E.; Lapina, T.V.; Sukhova, N.M.; Zidermane, A.A.; Dauvarte, A.Z.; Voronova, V.A. Nitrogen-containing organosilicon compounds. CIV. Synthesis and antiblastic activity of amides of quinoline carboxylic acids. Pharm. Chem. J. 1981, 15, 792–794. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–677. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chullay, J.D. Quantitative assessment of antimalarial activity in vitro by semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 3,15–22 (Table 1) are available from the authors. |

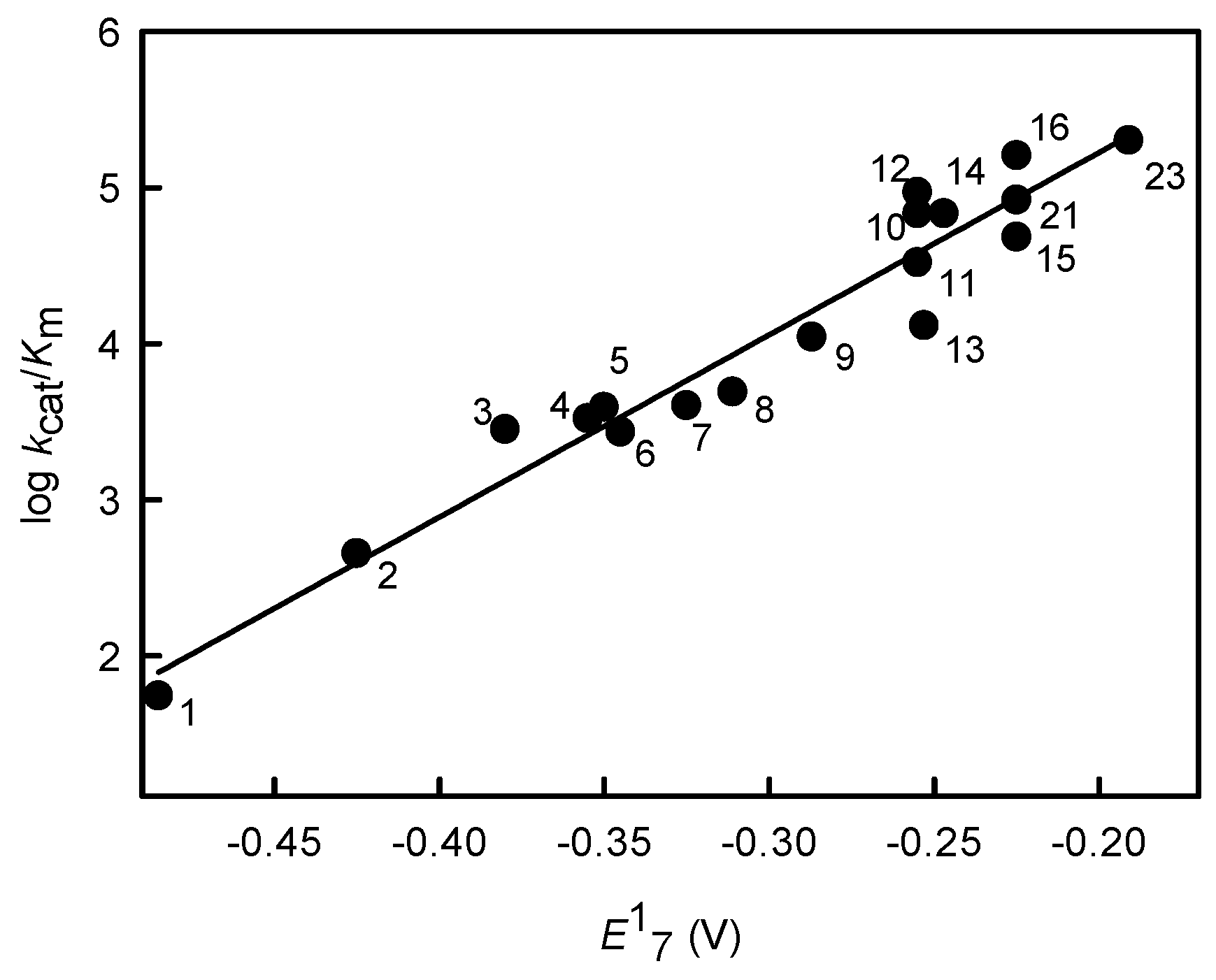
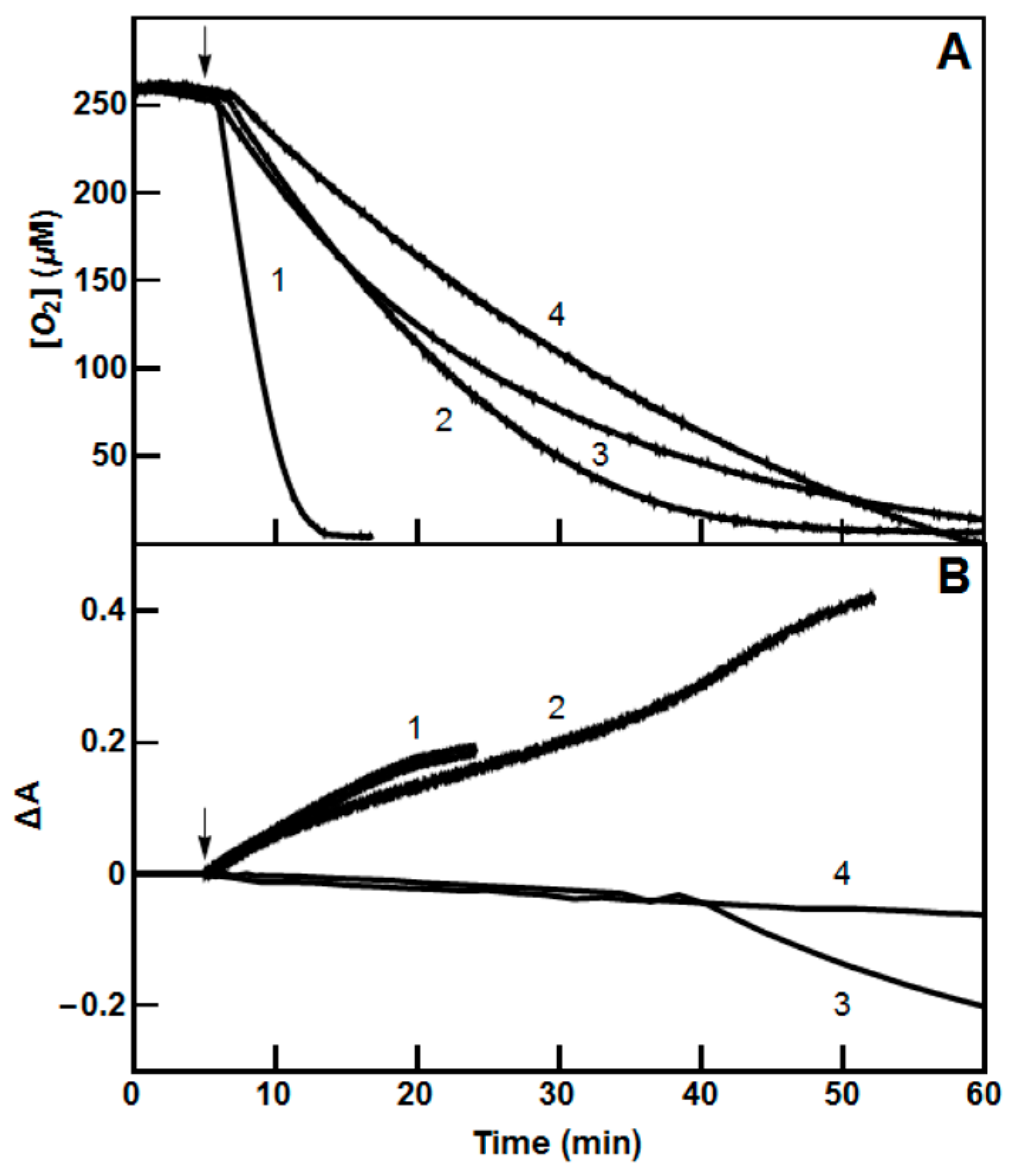
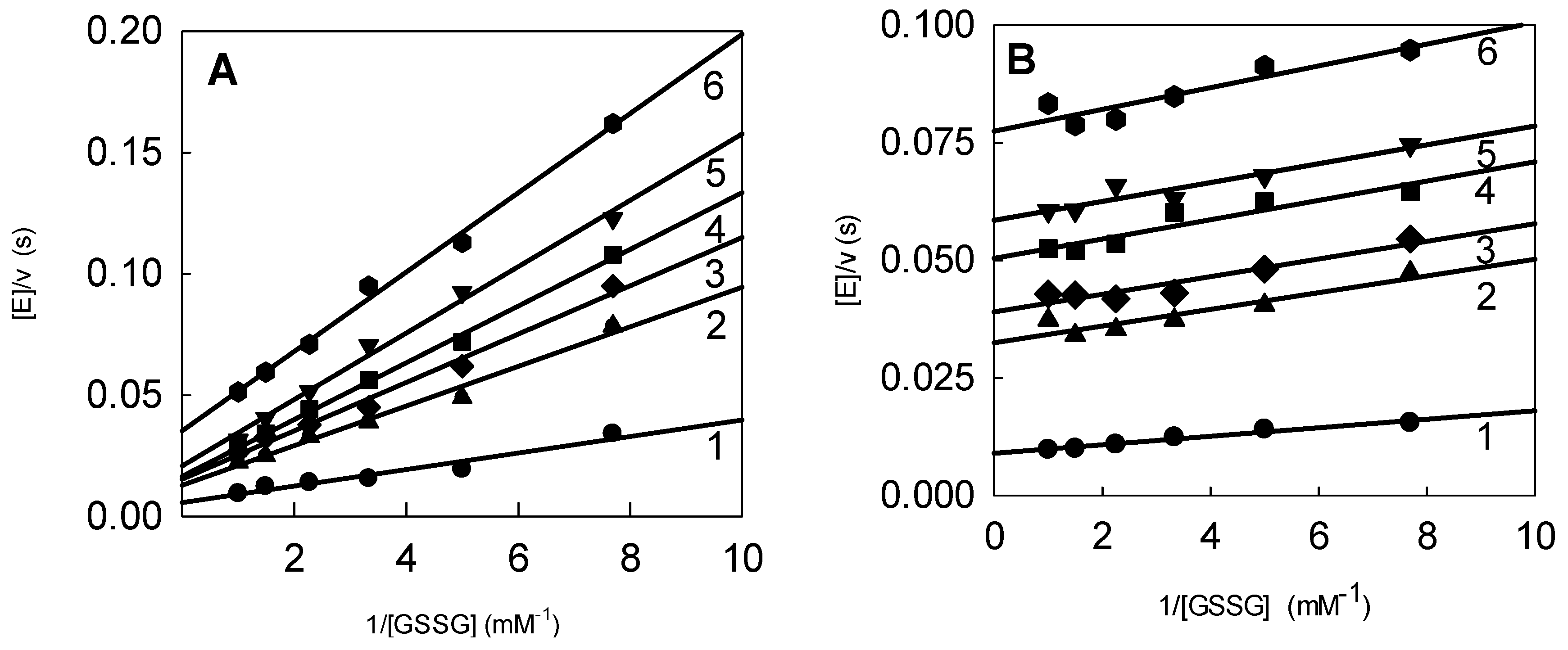

| No. | Compound | E17 (V) [35] | IC50 (µM) [19] | log D | kcat/Km (M−1·s−1) |
|---|---|---|---|---|---|
| 1 | Nitrobenzene | −0.485 | 473 ± 113 | 1.91 | 5.5 ± 0.8 × 101 |
| 2 | 4-Nitrobenzoic acid | −0.425 | 360 ± 16; 450 ± 70.7 b | −1.66 | 4.5 ± 0.6 × 102 |
| 3 | CB-1954 | −0.380 | 48.5 ± 5.0 b | 0.64 | 2.8 ± 0.3 × 103 |
| 4 | 4-Nitroacetophenone | −0.355 | 172 ± 8.0 | 1.47 | 3.3 ± 0.3 × 103 |
| 5 | 3,5-Dinitrobenzoic acid | −0.350 | 390 ± 17 | −1.79 | 3.9 ± 0.3 × 103 |
| 6 | 1,3-Dinitrobenzene | −0.345 | 50.5 ± 2.4 | 1.85 | 2.7 ± 0.3 × 103 |
| 7 | 4-Nitrobenzaldehyde | −0.325 | 79 ± 28 | 1.63 | 4.0 ± 0.3 × 103 |
| 8 | 3,5-Dinitrobenzamide | −0.311 | 30.3 ± 3.1; 26.5 ± 6.4 b | 0.7 | 4.9 ± 0.4 × 103 |
| 9 | 1,2-Dinitrobenzene | −0.287 | 11.7 ± 1.1 | 1.85 | 1.1 ± 0.2 × 104 |
| 10 | Nitrofurantoin | −0.255 | 12.9 ± 1.3 | −0.25 | 6.8 ± 0.7 × 104 |
| 11 | Nifuroxime | −0.255 | 14.7 ± 0.8 | −0.34 | 3.3 ± 0.4 × 104 |
| 12 | 1,4-Dinitrobenzene | −0.255 | 0.26 ± 0.03 | 1.85 | 9.3 ± 0.8 × 104 |
| 13 | 2,4,6-Trinitrotoluene | −0.253 | 9.4 ± 7.8 b | 2.31 | 1.3 ± 0.1 × 104 |
| 14 | N-Methylpicramide | −0.247 | 7.3 ± 1.1 b | 1.92 | 6.8 ± 0.5 × 104 |
| 15 | Nitrofuran IIIa | −0.225 a | 17.1 ± 1.5 | 0.27 | 4.8 ± 0.5 × 104 |
| 16 | Nitrofuran IIIb | −0.225 a | 4.5 ± 0.3 | 2.64 | 1.6 ± 0.2 × 105 |
| 17 | Nitrofuran IIIc | −0.225 a | 7.4 ± 0.3 | 2.87 | n.d. |
| 18 | Nitrofuran IIId | −0.225 a | 7.4 ± 0.3 | 3.23 | n.d. |
| 19 | Nitrofuran IIIe | −0.225 a | 9.2 ± 0.3 | 2.24 | n.d. |
| 20 | Nitrofuran IIIf | −0.225 a | 11.1 ± 0.4 | 2.62 | n.d. |
| 21 | Nitrofuran IIIg | −0.225 a | 6.4 ± 0.4 | 2.45 | 8.3 ± 0.7 × 104 |
| 22 | Nitrofuran IIIh | −0.225 a | 4.3 ± 0.3 | 2.62 | n.d. |
| 23 | Tetryl | −0.191 | 4.1 ± 0.8 b | 1.38 | 2.0 ± 0.3 × 105 |
| No. | Compound | Ki (μM) | |
|---|---|---|---|
| PfGR a | HGR b | ||
| 1 | Nitrobenzene | ≥6000 | ≥2000 |
| 2 | 4-Nitrobenzoic acid | 1200 ± 180 | 800 |
| 3 | CB-1954 | 350 ± 40 | ≥1000 |
| 4 | 4-Nitroacetophenone | 70 ± 11 | 400 |
| 5 | 3,5-Dinitrobenzoic acid | 220 ± 29 | 350 |
| 6 | 1,3-Dinitrobenzene | 40 ± 5.0 | 320; 350 ± 30a |
| 7 | 4-Nitrobenzaldehyde | 25 ± 4.0 | 290 |
| 8 | 3,5-Dinitrobenzamide | 75 ± 9.0 | ≥1000 |
| 9 | 1,2-Dinitrobenzene | 30 ± 4.0 | ≥1000 |
| 10 | Nitrofurantoin | 9.0 ± 1.0 | 200 |
| 11 | Nifuroxime | 32 ± 5.0 | 200 |
| 12 | 1,4-Dinitrobenzene | 0.85 ± 0.13 | 71 |
| 13 | 2,4,6-Trinitrotoluene | 8.0 ± 2.0 | 6.0 c; 5.2 ± 0.6 a |
| 14 | N-Methylpicramide | 5.9 ± 0.6 | 10 c |
| 15 | Nitrofuran IIIa | 9.0 ± 1.0 | 3.0; 3.5 ± 0.2 a |
| 16 | Nitrofuran IIIb | 25 ± 3.0 | 2.5 |
| 17 | Nitrofuran IIIc | 115 ± 17 | 25 |
| 18 | Nitrofuran IIId | 50 ± 6.0 | ≥300 |
| 19 | Nitrofuran IIIe | 5.0 ± 1.0 | 2.5 |
| 20 | Nitrofuran IIIf | 75 ± 10 | 42.5 |
| 21 | Nitrofuran IIIg | 35 ± 5.0 | 25 |
| 22 | Nitrofuran IIIh | 100 ± 12 | 45 |
| 23 | Tetryl | 2.3 ± 0.5 | 14 c |
| Enzyme | Oxidants | |
|---|---|---|
| Nitrofurans, Nitrobenzenes | Menadione | |
| E17 = −0.25–−0.19 V | ||
| PfFNR | kcat > 20 s−1, kcat/Km = 4.8 × 104 – 1.6×105 M−1·s−1, this work | kcat = 14 s−1, kcat/Km = 1.0 ×106 M−1·s−1 [30] |
| PfGR | kcat = 0.06–5.9 s−1, kcat/Km = 7.6 × 103 – 110 M−1·s−1, this work | kcat = 0.16 s−1, kcat/Km = 2.0 × 103 M−1·s−1 [54] |
| P. falciparum thioredoxin reductase | kcat = 31 s−1, kcat/Km = 1.6 × 105 M−1·s−1 [54] | |
| P. falciparum type II NADH dehydrogenase | kcat = 0.1 s−1 [55] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marozienė, A.; Lesanavičius, M.; Davioud-Charvet, E.; Aliverti, A.; Grellier, P.; Šarlauskas, J.; Čėnas, N. Antiplasmodial Activity of Nitroaromatic Compounds: Correlation with Their Reduction Potential and Inhibitory Action on Plasmodium falciparum Glutathione Reductase. Molecules 2019, 24, 4509. https://doi.org/10.3390/molecules24244509
Marozienė A, Lesanavičius M, Davioud-Charvet E, Aliverti A, Grellier P, Šarlauskas J, Čėnas N. Antiplasmodial Activity of Nitroaromatic Compounds: Correlation with Their Reduction Potential and Inhibitory Action on Plasmodium falciparum Glutathione Reductase. Molecules. 2019; 24(24):4509. https://doi.org/10.3390/molecules24244509
Chicago/Turabian StyleMarozienė, Audronė, Mindaugas Lesanavičius, Elisabeth Davioud-Charvet, Alessandro Aliverti, Philippe Grellier, Jonas Šarlauskas, and Narimantas Čėnas. 2019. "Antiplasmodial Activity of Nitroaromatic Compounds: Correlation with Their Reduction Potential and Inhibitory Action on Plasmodium falciparum Glutathione Reductase" Molecules 24, no. 24: 4509. https://doi.org/10.3390/molecules24244509






