Antidepressant Potential of Cinnamic Acids: Mechanisms of Action and Perspectives in Drug Development
Abstract
:1. Introduction
2. Materials and Methods
3. Antidepressant Activity of Cinnamic Acids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-Hydroxyindoleacetic acid |
| 5-HT | Serotonin |
| 5-LOX | 5-Lipoxygenase |
| BDNF | Brain-derived neurotrophic factor |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| EPM | Elevated plus maze test |
| FST | Forced swim test |
| IL-1β | Interleukin-1β |
| IL-8 | Interleukin-8 |
| LPS | Lipopolysaccharide |
| MAO | Monoamine oxidase |
| MAO-A | Monoamine oxidase A |
| MAPK/ERK | Mitogen-activated protein kinases/extracellular signal-regulated kinases |
| MHPG | 3-Methoxy-4-hydroxyphenylglycol |
| NF-κB | Nuclear factor kappa B |
| NE | Norepinephrine |
| NET | Norepinephrine transporter |
| NO | Nitric oxide |
| OFT | Open field test |
| PI3K | Phosphoinositide 3-kinases |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| ROS | Reactive species of oxygen |
| SERT | Serotonin transporter |
| SNRI | Serotonin norepinephrine reuptake inhibitors |
| SOD | Superoxide dismutase |
| SSRI | Selective serotonin reuptake inhibitor |
| SST | Sucrose splash test |
| TBARS | Thiobarbituric acid reactive substances |
| TCA | Tricyclic antidepressants |
| TYR | Tyrosine |
| TMCA | 2,4,5-Trimethoxycinnamic acid |
| TNF-α | Tumor necrosis factor-α |
| TRP | Tryptophan |
| TST | Tail suspension test |
References
- Peres, M.F.P.; Mercante, J.P.P.; Tobo, P.R.; Kamei, H.; Bigal, M.E. Anxiety and depression symptoms and migraine: A symptom-based approach research. J. Headache Pain 2017, 18, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef] [PubMed]
- Bravender, T. Mental Disorders and Learning Disabilities in Children and Adolescents: Depression in Adolescents. FP Essent. 2018, 475, 30. [Google Scholar] [PubMed]
- Wang, S.; Blazer, D.G. Depression and cognition in the elderly. Annu Rev. Clin. Psychol. 2015, 11, 331. [Google Scholar] [CrossRef]
- Gournellis, R.; Tournikioti, K.; Touloumi, G.; Thomadakis, C.; Michalopoulou, P.G.; Michopoulos, I.; Christodoulou, C.; Papadopoulou, A.; Douzenis, A. Psychotic (delusional) depression and completed suicide: A systematic review and meta-analysis. Ann. Gen. Psychiatry 2018, 17, 39. [Google Scholar] [CrossRef] [Green Version]
- Hawton, K.; Casanas, I.C.C.; Haw, C.; Saunders, K. Risk factors for suicide in individuals with depression: A systematic review. J. Affect. Disord. 2013, 147, 17. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894. [Google Scholar] [CrossRef]
- Peng, G.J.; Tian, J.S.; Gao, X.X.; Zhou, Y.Z.; Qin, X.M. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, N.A.L.; Del Angel, D.S.; Olguin, H.J.; Silva, M.L. Neuroprogression: The hidden mechanism of depression. Neuropsychiatr. Dis. Treat. 2018, 14, 2837. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Yamagata, H.; Seki, T.; Watanabe, Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin. Neurosci 2018, 72, 212. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.H.; Zhang, G.Z.; Li, B.; Li, M.; Woelfer, M.; Walter, M.; Wang, L. Role of inflammation in depression relapse. J. Neuroinflammation 2019, 16, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reus, G.Z.; Silva, R.H.; de Moura, A.B.; Presa, J.F.; Abelaira, H.M.; Abatti, M.; Vieira, A.; Pescador, B.; Michels, M.; Ignacio, Z.M.; et al. Early Maternal Deprivation Induces Microglial Activation, Alters Glial Fibrillary Acidic Protein Immunoreactivity and Indoleamine 2,3-Dioxygenase during the Development of Offspring Rats. Mol. Neurobiol. 2019, 56, 1096. [Google Scholar] [CrossRef] [PubMed]
- Oglodek, E.A. Changes in the concentrations of inflammatory and oxidative status biomediators (MIP-1 alpha, PMN elastase, MDA, and IL-12) in depressed patients with and without posttraumatic stress disorder. Pharmacol Rep. 2018, 70, 110. [Google Scholar] [CrossRef] [PubMed]
- Stanners, M.N.; Barton, C.A.; Shakib, S.; Winefield, H.R. Depression diagnosis and treatment amongst multimorbid patients: A thematic analysis. BMC Fam. Pract. 2014, 15, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, T.P. Depressive disorders: Treatment failures and poor prognosis over the last 50 years. Pharmacol. Res. Perspect. 2019, 7, e00472. [Google Scholar] [CrossRef] [PubMed]
- Hollon, S.D.; Cohen, Z.D.; Singla, D.R.; Andrews, P.W. Recent Developments in the Treatment of Depression. Behav. Ther. 2019, 50, 257. [Google Scholar] [CrossRef]
- López-Rubalcava, C.; Estrada-Camarena, E. Mexican medicinal plants with anxiolytic or antidepressant activity: Focus on preclinical research. J. Ethnopharmacol. 2016, 20, 377–391. [Google Scholar]
- Guzman, J.D.; Mortazavi, P.N.; Munshi, T.; Evangelopoulos, D.; Mchugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. 2-Hydroxy-substituted cinnamic acids and acetanilides are selective growth inhibitors of Mycobacterium tuberculosis. MedChemComm 2014, 5, 47–50. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, W.; Lu, Y.; Wang, Y.; Chen, X.; Bai, S.; Zhao, Y.; He, T.; Lao, F.; Shang, Y.; et al. Naturally Occurring Cinnamic Acid Sugar Ester Derivatives. Molecules 2016, 21, 1402. [Google Scholar] [CrossRef] [Green Version]
- de Cássia, D.S.E.S.; Andrade, L.N.; Dos Reis, B.D.O.R.; de Sousa, D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef] [Green Version]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Hu, Y.; Guo, D.H.; Wang, D.X.; Tu, H.H.; Ma, L.; Xie, T.T.; Kong, L.Y. Potential antidepressant properties of Radix polygalae (Yuan Zhi). Phytomedicine 2010, 17, 794–799. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Magoulas, G.E.; Papaioannou, D. Bioinspired syntheses of dimeric hydroxycinnamic acids (lignans) and hybrids, using phenol oxidative coupling as key reaction, and medicinal significance thereof. Molecules 2014, 19, 19769. [Google Scholar] [CrossRef] [Green Version]
- Bialecka-Florjanczyk, E.; Fabiszewska, A.; Zieniuk, B. Phenolic Acids Derivatives-Biotechnological Methods of Synthesis and Bioactivity. Curr. Pharm. Biotechnol. 2018, 19, 1098. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Ren, F.; Qin, Y.; Liu, J.; Liu, J.; Wang, Q.; Zhang, H. Structure-affinity relationship of the interaction between phenolic acids and their derivatives and beta-lactoglobulin and effect on antioxidant activity. Food Chem. 2018, 245, 613. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653. [Google Scholar] [CrossRef]
- Gaspar, A.; Garrido, E.M.; Esteves, M.; Quezada, E.; Milhazes, N.; Garrido, J.; Borges, F. New insights into the antioxidant activity of hydroxycinnamic acids: Synthesis and physicochemical characterization of novel halogenated derivatives. Eur. J. Med. Chem. 2009, 44, 2092. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013, 20, 4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagne, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011, 55, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327. [Google Scholar] [CrossRef] [Green Version]
- Abelaira, H.M.; Reus, G.Z.; Quevedo, J. Animal models as tools to study the pathophysiology of depression. Braz. J. Psychiatry 2013, 35, S112–S120. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Leem, Y.H.; Oh, S. 3, 4, 5-Trimethoxycinnamin acid ameliorates restraint stress-induced anxiety and depression. Neurosci. Lett. 2015, 585, 54–59. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Inazu, M.; Egashira, T.; Matsumiya, T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002, 449, 261–267. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Miyamoto, J.; Masuya, J.; Iimori, M.; Matsumiya, T. Caffeic acid produces antidepressive-and/or anxiolytic-like effects through indirect modulation of the α1A-adrenoceptor system in mice. Neuroreport 2003, 14, 1067–1070. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Yamada, T.; Masuya, J.; Matsushita, K.; Tahara, M.; Matsumiya, T.; Limore, M.; Matsumiya, T. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur. J. Pharmacol. 2006, 534, 115–121. [Google Scholar] [CrossRef]
- Dzitoyeva, S.; Imbesi, M.; Uz, T.; Dimitrijevic, N.; Manev, H.; Manev, R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J. Neural Transm. Suppl. 2008, 115, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, L.; Yang, J.Q.; Luo, Y.; Cui, T.; Du, T.T.; Jiang, X.H. Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid. Genes Dis. 2019, 6, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332. [Google Scholar] [CrossRef] [PubMed]
- Zeni, A.L.B.; Zomkowski, A.D.E.; Maraschin, M.; Rodrigues, A.L.S.; Tasca, C.I. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur. J. Pharmacol. 2012, 679, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Zeni, A.L.B.; Camargo, A.; Dalmagro, A.P. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 2017, 125, 131–136. [Google Scholar] [CrossRef]
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, K.; Li, J.; Yu, X.; Xie, X.; et al. Antidepressant-like effects of ferulic acid: Involvement of serotonergic and norepinergic systems. Metab. Brain Dis. 2015, 30, 129–136. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, X.; Wang, Y.; Xie, Y.; Qiu, X.J.; Ren, P.; Gao, L.C.; Zhou, H.H.; Zhange, H.Y.; Qiao, M.Q. Ferulic acid-induced anti-depression and prokinetics similar to Chaihu–Shugan–San via polypharmacology. Brain Res. Bull. 2011, 86, 222–228. [Google Scholar] [CrossRef]
- Sasaki, K.; Iwata, N.; Ferdousi, F.; Isoda, H. Antidepressant-Like Effect of Ferulic Acid via Promotion of Energy Metabolism Activity. Mol. Nutr. Food Res. 2019, 63, 1900327. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, J.; Rodrigues, A.F.; de Sousa Rós, A.; de Castro, B.B.; de Lima, D.D.; Dal Magro, D.D.; Zeni, A.L.B. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef]
- Li, G.; Ruan, L.; Chen, R.; Wang, R.; Xie, X.; Zhang, M.; Chen, L.; Yan, Q.; Reed, M.; Chen, J.; et al. Synergistic antidepressant-like effect of ferulic acid in combination with piperine: Involvement of monoaminergic system. Metab. Brain Dis. 2015, 30, 1505–1514. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.M.; Hu, C.Y.; Shen, J.D.; Wu, S.H.; Li, Y.C.; Yi, L.T. Elevation of synaptic protein is associated with the antidepressant-like effects of ferulic acid in a chronic model of depression. Physiol. Behav. 2017, 169, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cheng, Y.; Chen, Y.; Yue, Y.; Li, Y.; Xia, S.; Li, Y.; Deng, H.; Zang, J.; Cao, Y. Ferulic Acid Improves Depressive-Like Behavior in Prenatally-Stressed Offspring Rats via Anti-Inflammatory Activity and HPA Axis. Int. J. Mol. Sci. 2019, 20, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, H.B.; Hwang, E.S.; Kim, E.S.; Kim, S.S.; Jeon, T.D.; Song, M.; Li, J.S.; Chung, M.C.; Maeng, S.; et al. Antidepressant-like effects of p-coumaric acid on LPS-induced depressive and inflammatory changes in rats. Exp. Neurobiol. 2018, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Barauna, S.C.; Delwing-Dal, M.D.; Brueckheimer, M.B.; Maia, T.P.; Sala, G.A.B.N.; Döhler, A.W.; Harger, M.C.; de Melo, D.F.M.; de Gasper, A.L.; Alberton, M.D.; et al. Antioxidant and antidepressant-like effects of Eugenia catharinensis D. Legrand in an animal model of depression induced by corticosterone. Metab. Brain Dis. 2018, 33, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Adeoluwa, A.O.; Aderibigbe, O.A.; Agboola, I.O.; Olonode, T.E.; Ben-Azu, B. Butanol Fraction of Olax subscorpioidea Produces Antidepressant Effect: Evidence for the Involvement of Monoaminergic Neurotransmission. Drug Res. (Stuttg). 2019, 69, 53–60. [Google Scholar] [CrossRef]
- Pauleti, N.N.; Mello, J.; Siebert, D.A.; Micke, G.A.; de Albuquerque, C.A.C.; Alberton, M.; Barauna, S.C. Characterisation of phenolic compounds of the ethyl acetate fraction from Tabernaemontana catharinensis and its potential antidepressant-like effect. Nat. Prod. Res. 2018, 32, 1987–1990. [Google Scholar] [CrossRef]
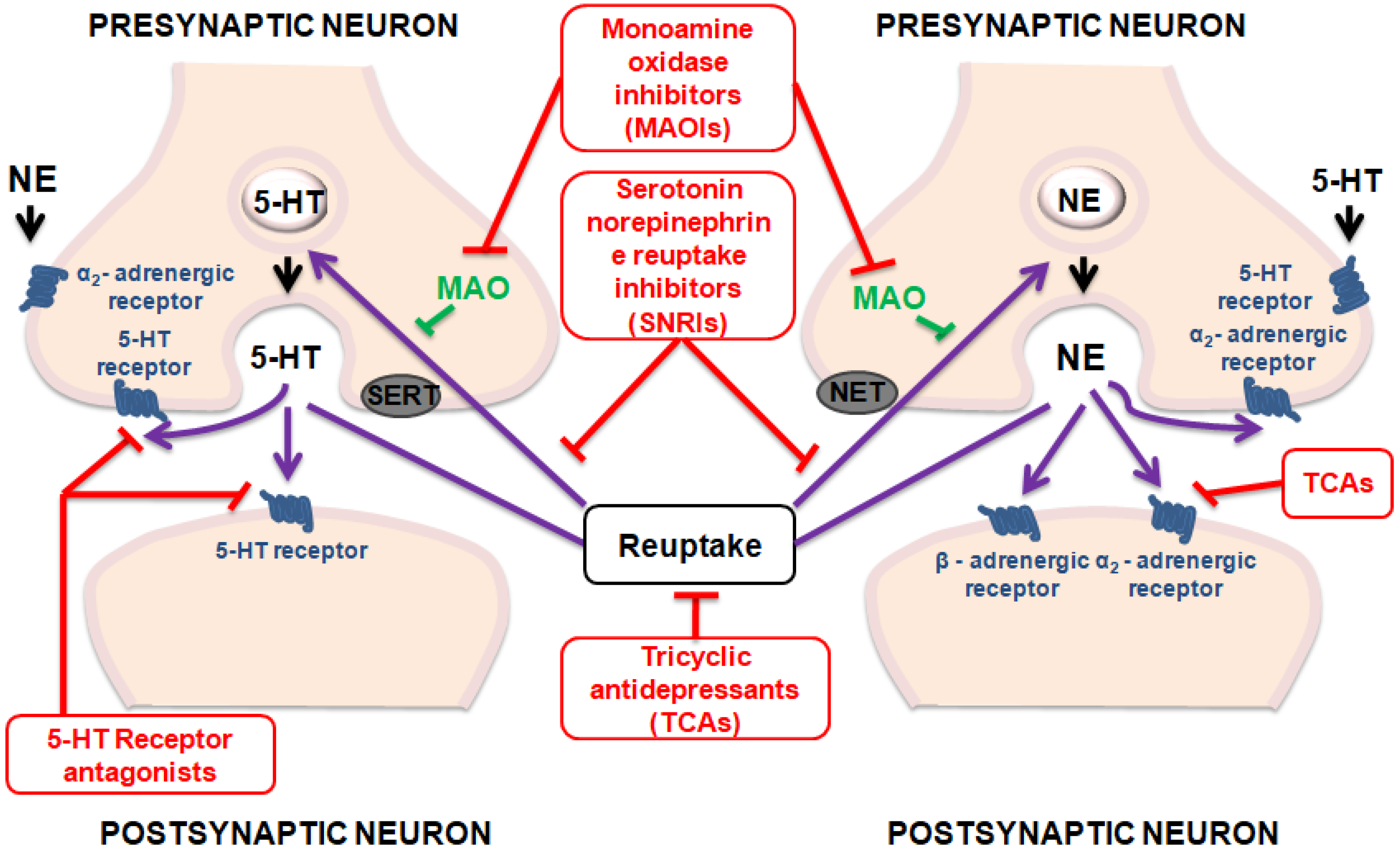
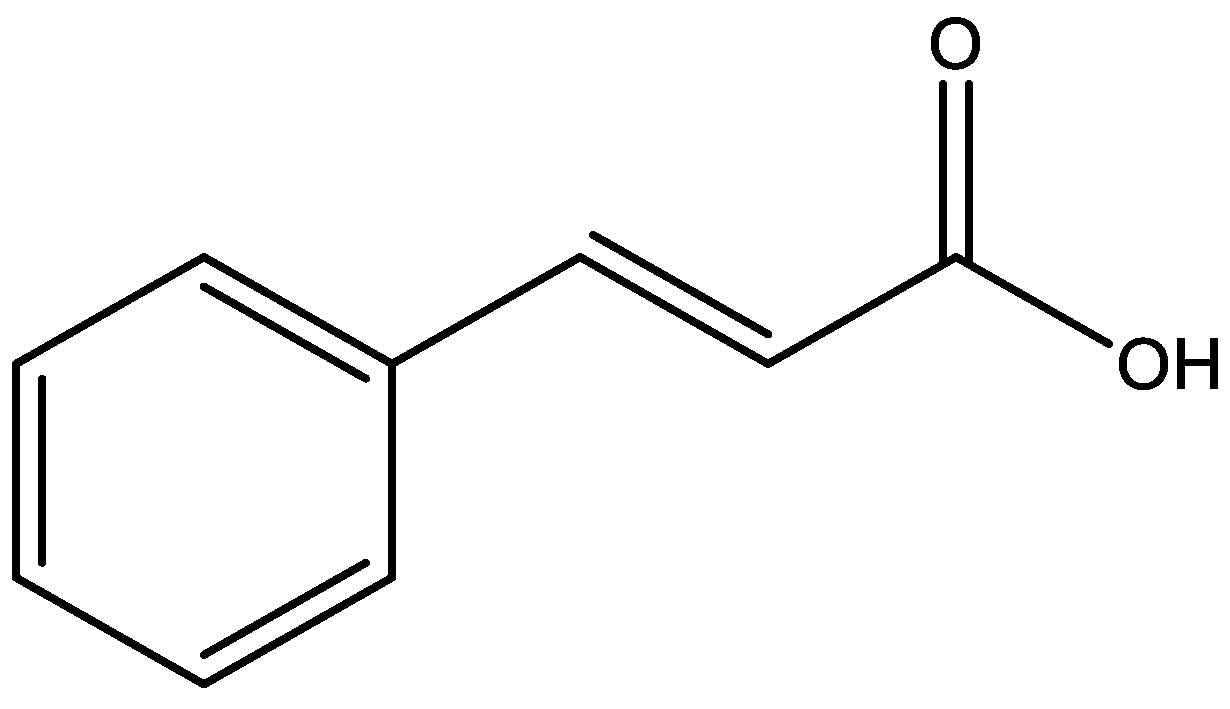
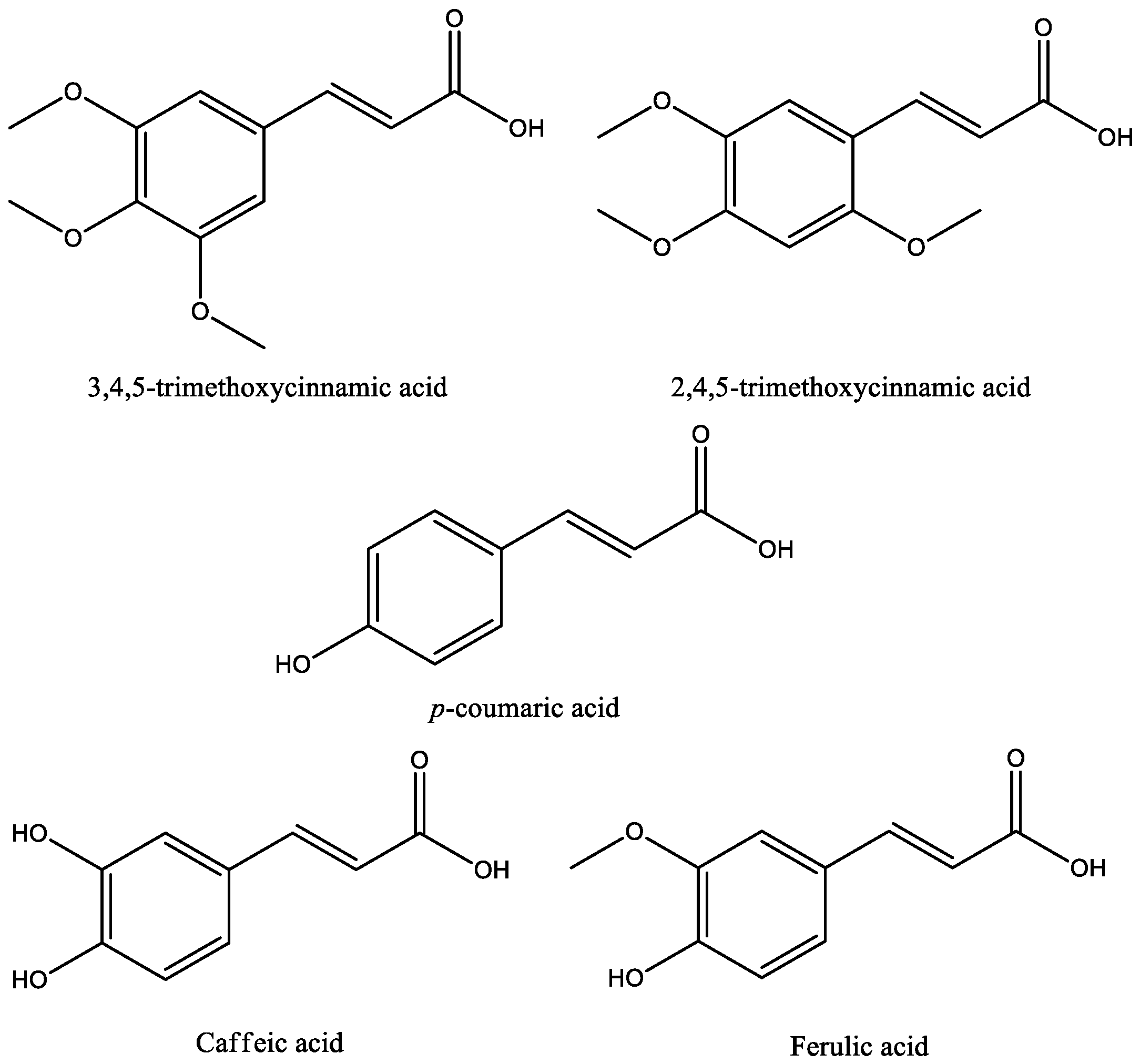
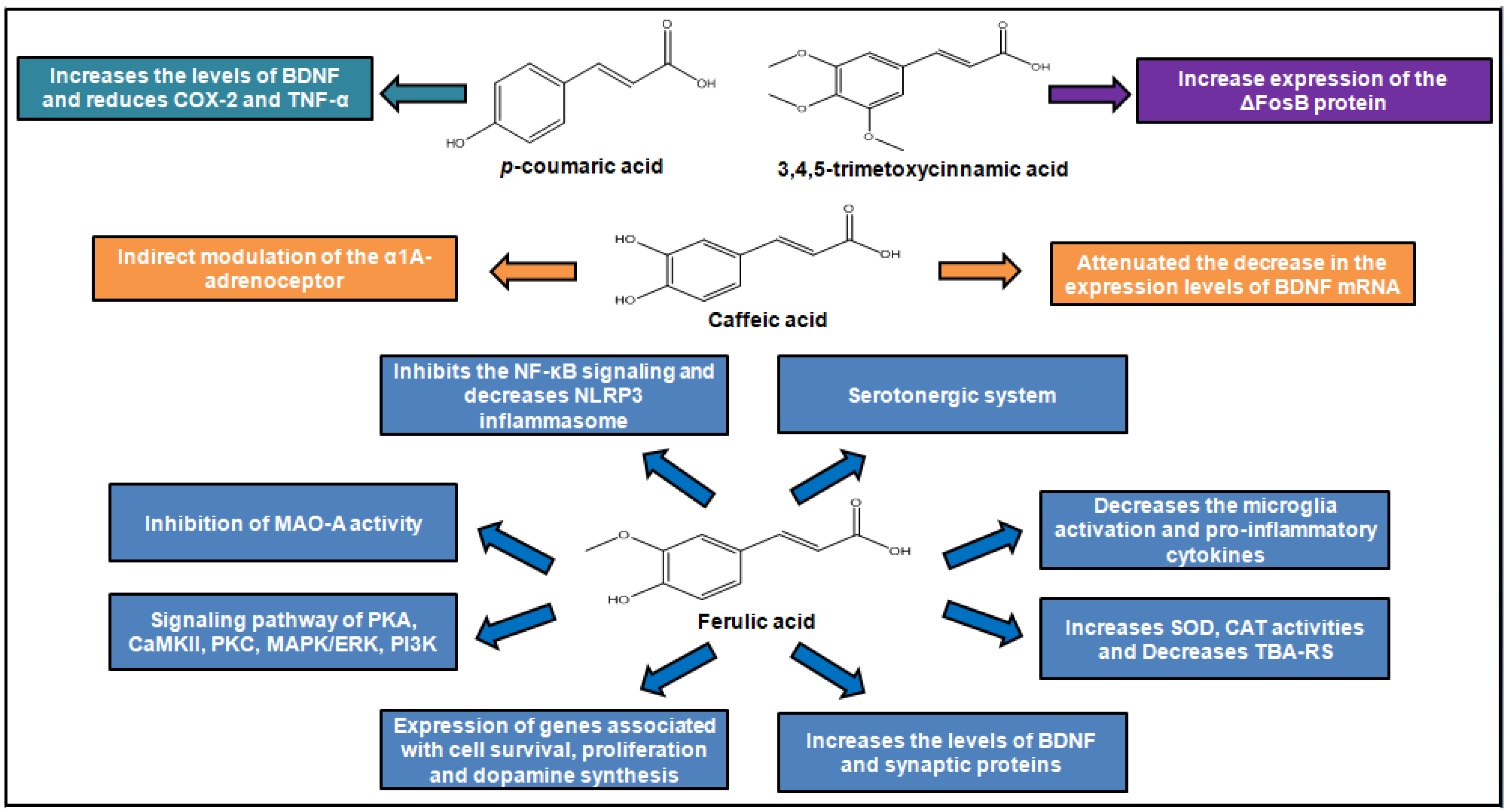
| Compound | Animal Species | Dose and via of Administration | Behavioral Test | Observed Effects | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| 2,4,5-Trimethoxy- cinnamic acid (TMCA) | Male ddY mice | 25, 50, 100 and 200 mg/kg, i.p. | FST | The treatment failed to alter the duration of immobility | - | [36] |
| 3,4,5-Trimethoxy- cinnamic acid (TMCA) | Male C57BL/6J mice | 25 and 50 mg/kg, p.o. | EPM; FST | The dose 50 mg/kg of treatment increased time and frequency of visits in the open arms of the EPM and showed reduced immobility in the FST | Increase expression of the ΔFosB protein on the nucleus accumbens | [37] |
| Caffeic acid | Male ICR mice | 1, 2 and 4 mg/kg, i.p. | FST; spontaneous motor activity | The dose 4.0 mg/kg reduced the duration of immobility of mice | - | [38] |
| Caffeic acid | Male ICR mice and ddY mice | 4 mg/kg,i.p. | Conditioned fear stress test | Reduced the duration of immobility of mice in the forced swimming test and reduced the duration of freezing of mice in the conditioned fear stress test | Indirect modulation of the α1A-adrenoceptor and α1-adrenoceptor system | [39] |
| Caffeic acid | Male ICR mice | 4 mg/kg,i.p. | FST | Reduced the duration of immobility of mice in the forced swimming test | Attenuated the decrease in the expression levels of BDNF mRNA in the frontal cortex of mice following forced swimming | [40] |
| Caffeic acid | Male 5-LOX deficient mice and wild type | 4 mg/kg,i.p. | FST | The pre-treatment was able to attenuate this decrease in the wild-type group | Caffeic acid can be used as a tool to study 5-lipoxygenase (5-LOX) pathway regulation of brain-derived neurotrophic factor (BDNF) expression | [41] |
| Caffeic acid | Male Sprague-Dawley rats | 50, 75 and 100 mg/kg, i.p | OFT; FST | Inhibited the decrease of NE and the increase of Trp and MHPG in a dose-dependent manner | The inhibition of AA-COX-2/5-LOX pathways can improve the behaviors of depression rats | [42] |
| Ferulic acid | Male Sprague–Dawley rats | 25 and 50 mg/kg, p.o. | FST; OFT | The dose 50 mg/kg reduced the duration of immobility of mice | Involvement of serotonergic system | [47] |
| Ferulic acid | Male Swiss mice | 0.001, 0.01, 0.1, 1 and 10 mg/kg, p.o. | FST; TST; OFT | The doses 0.01, 0.1, 1 and 10 mg/kg reduced the duration of immobility of mice | Involvement of serotonergic system | [44] |
| Ferulic acid | Male Swiss mice | 0.01 mg/kg, p.o. | TST; OFT | The dose 0.01 mg/kg reduced the duration of immobility of mice | Involvement signaling pathway of PKA, CaMKII, PKC, MAPK/ERK and PI3K | [44] |
| Ferulic acid | Male ICR mice | 10, 20, 40 and 80 mg/kg, p.o. | FST; TST | The doses 40 and 80 mg/kg showed reduced immobility in the tests | The increase on the concentrations of monoamines 5-HT and norepinephrine in the hippocampus and frontal cortex through inhibition monoamine oxidase A (MAO-A) activity | [46] |
| Ferulic acid | Male Swiss mice | 0.01, 0.1, 1 and 10 mg/kg/day, p.o. | FST; TST; OFT | The dose 1.0 mg/kg reduced the duration of immobility of mice | Increases SOD, CAT activities and decreases TBA-RS levels in hippocampus | [49] |
| Ferulic acid | Male ICR mice | 3, 10, 30 and 90 mg/kg, p.o. | TST; FST; Locomotor activity | All doses reduced the duration of immobility of mice | - | [50] |
| Ferulic acid | Male ICR mice | 20 and 40 mg/kg, p.o. | FST | The dose 40 mg/kg reduced the duration of immobility of mice | Increased the levels of BDNF and synaptic proteins (synapsin I and PSD-95) in both the prefrontal cortex and hippocampus. | [51] |
| Ferulic acid | Male ICR mice | 20, 40 or 80 mg/kg, p.o. | SST; TST | All doses reduced the duration of immobility of mice | Inhibition of the microglia activation, pro-inflammatory cytokines expression, NF-κB signaling and decreased NLRP3 inflammasome | [51] |
| Ferulic acid | Male Swiss mice | 1 mg/kg, p.o. | TST; OFT; SST | The dose 1.0 mg/kg reduced the duration of immobility of mice | - | [45] |
| Ferulic acid | Male ICR mice | 5 mg/kg, p.o | TST | The dose 5 mg/kg reduced the duration of immobility of mice | Upregulates the expression of several genes associated with cell survival and proliferation, energy metabolism, and dopamine synthesis in mice limbic system of brain | [48] |
| Ferulic acid | Male Sprague-Dawley rats prenatally | 12.5, 25, and 50 mg/kg, i.g. | SST, FST, OFT | Increased sucrose intake, and decreased immobility time and total number of crossings, rearing and grooming | Decreased concentration of inflammatory cytokines such as IL-6, IL-1 and TNF-α and increases IL-10 | [52] |
| p-Coumaric acid | Male Sprague-Dawley rats | 10 and 30 mg/kg p.o | FST; TST; SST | Improved LPS-induced despair-related behavioral symptoms | Prevented the increase of inflammatory cytokines in the hippocampus and the reduction of BDNF | [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, L.R.L.; Souza, M.T.d.S.; Barboza, J.N.; Almeida, R.N.d.; Sousa, D.P.d. Antidepressant Potential of Cinnamic Acids: Mechanisms of Action and Perspectives in Drug Development. Molecules 2019, 24, 4469. https://doi.org/10.3390/molecules24244469
Diniz LRL, Souza MTdS, Barboza JN, Almeida RNd, Sousa DPd. Antidepressant Potential of Cinnamic Acids: Mechanisms of Action and Perspectives in Drug Development. Molecules. 2019; 24(24):4469. https://doi.org/10.3390/molecules24244469
Chicago/Turabian StyleDiniz, Lúcio Ricardo Leite, Marilia Trindade de Santana Souza, Joice Nascimento Barboza, Reinaldo Nóbrega de Almeida, and Damião Pergentino de Sousa. 2019. "Antidepressant Potential of Cinnamic Acids: Mechanisms of Action and Perspectives in Drug Development" Molecules 24, no. 24: 4469. https://doi.org/10.3390/molecules24244469







