Secondary Metabolites of The Endophytic Fungus Alternaria alternata JS0515 Isolated from Vitex rotundifolia and Their Effects on Pyruvate Dehydrogenase Activity
Abstract
:1. Introduction
2. Results and Discussion
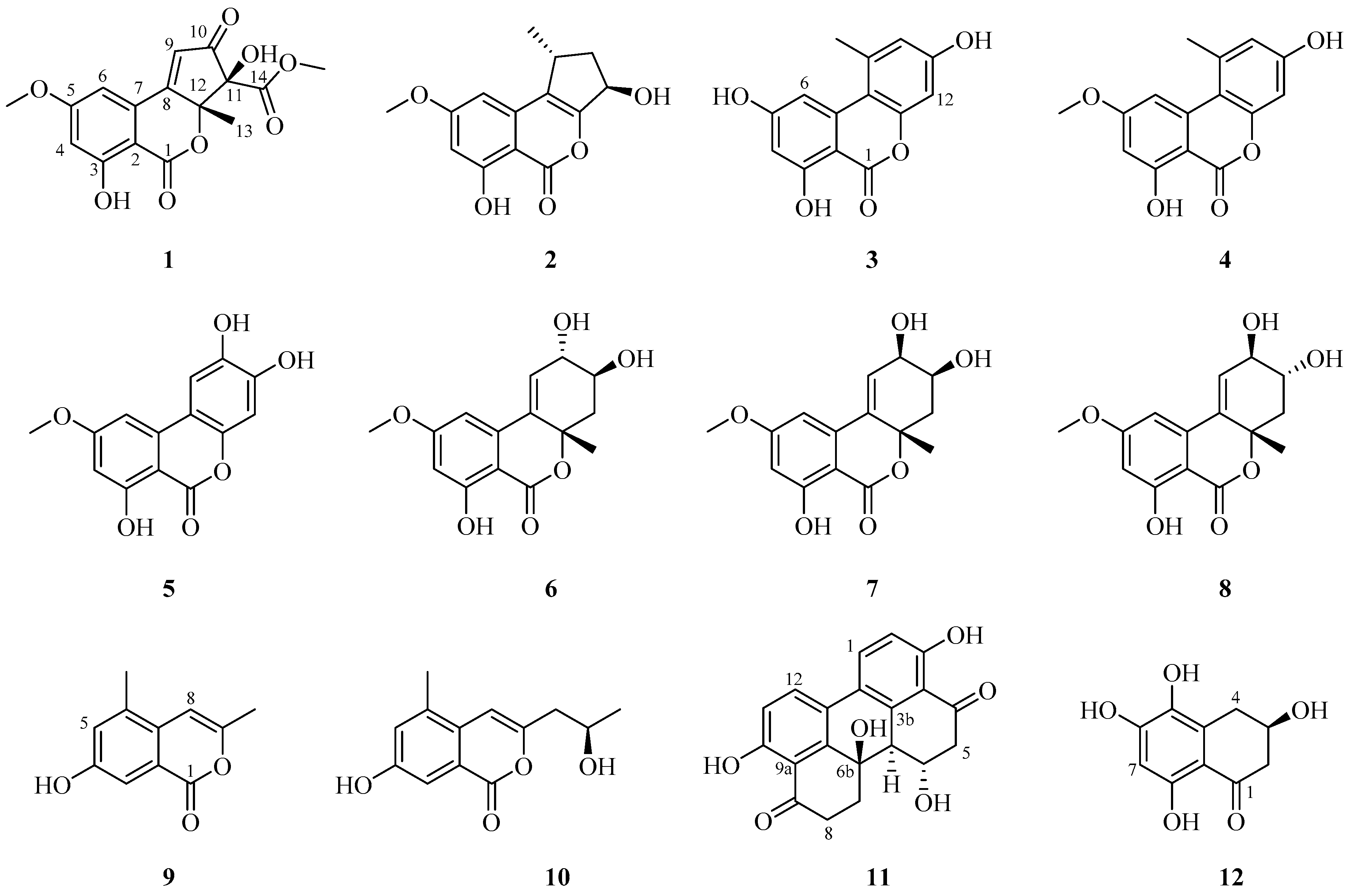
2.1. Isolation and Structural Elucidation
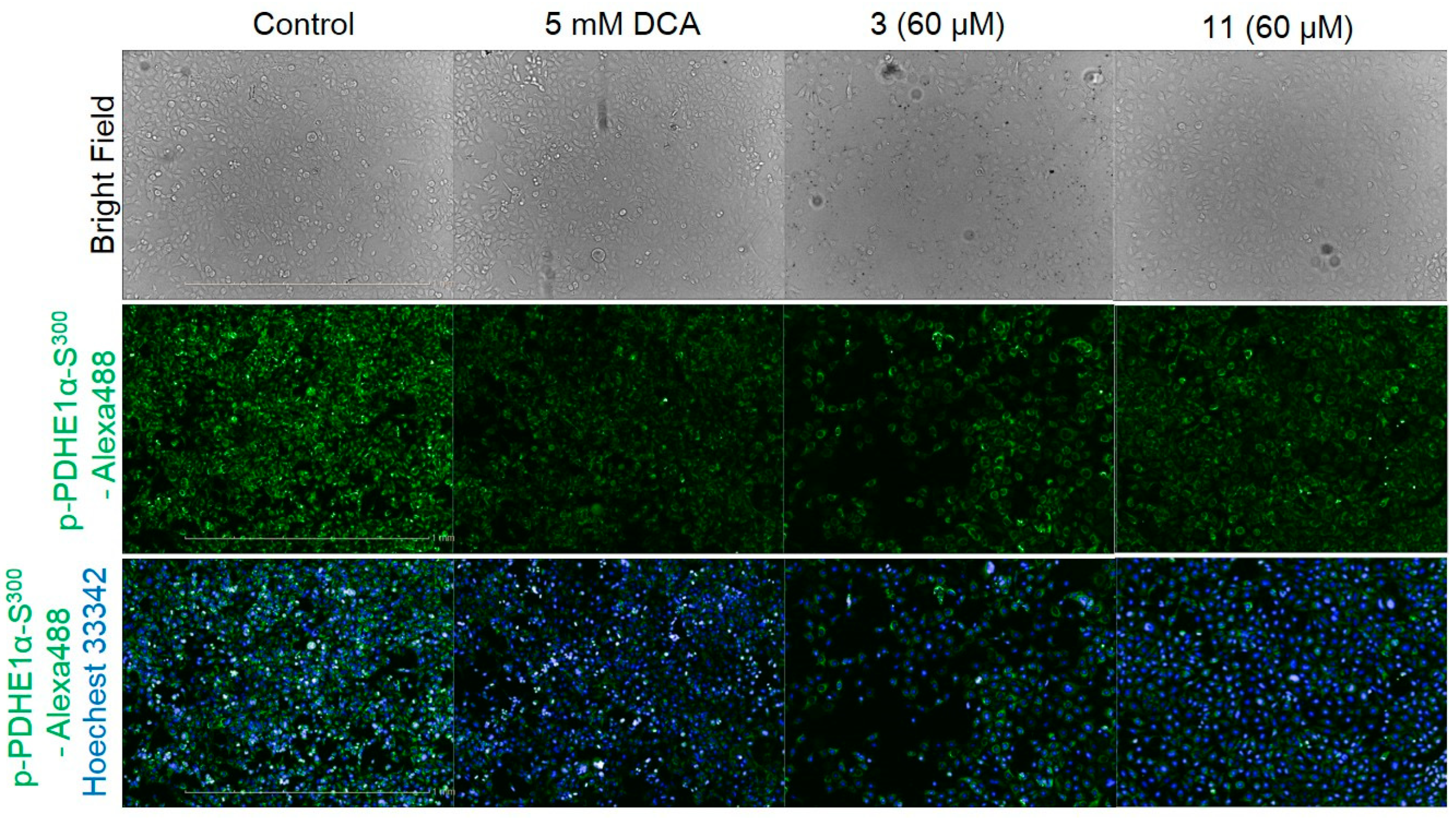
2.2. Bioassays
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Isolation of The Fungal Strain
4.3. Cultivation and Extraction of The Fungal Strain
4.4. Isolation of Secondary Metabolites
4.5. Pyruvate Dehydrogenase Complex (PDH) Cellular Activity
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Niimura, N. Determination of the type of lacquer on East Asian lacquer ware. Int. J. Mass Spectrom. 2009, 284, 93–97. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Mao, L.J.; Li, N.; Feng, X.X.; Yuan, Z.L.; Wang, L.W.; Lin, F.C.; Zhang, C.L. Evidence for biotrophic lifestyle and biocontrol potential of dark septate endophyte Harpophora oryzae to rice blast disease. PLoS ONE 2013, 8, e61332. [Google Scholar] [CrossRef] [Green Version]
- Pudhom, K.; Teerawatananond, T.; Rhytidenones, A.-F. Spirobisnaphthalenes from Rhytidhysteron sp. AS21B, an endophytic fungus. J. Nat. Prod. 2014, 77, 1962–1966. [Google Scholar] [CrossRef]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.M.; Teplow, D.B.; et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microniology 2002, 148, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Wedge, D.E.; Nagle, D.G. A new 2D-TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J. Nat. Prod. 2000, 63, 1050–1054. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.; Li, W.; Bang, S.; Lee, H.; Lee, H.; Noh, E.; Park, J.; Bang, W.Y.; Shim, S.H. Bioactive secondary metabolites produced by an endophytic fungus Gaeumannomyces sp. JS0464 from a maritime halophyte Phragmites communis. J. Antibiot. 2017, 70, 737–742. [Google Scholar] [CrossRef]
- Sgroy, V.; Casssan, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef]
- Kawazoe, K.; Yutani, A.; Tamemoto, K.; Yuasa, S.; Shibata, H.; Higuti, T.; Takaishi, Y. Phenylnaphthalene compounds from the subterranean part of Vitex rotundifolia and their antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2001, 64, 588–591. [Google Scholar] [CrossRef]
- Rani, A.; Sharma, A. The genus Vitex: A review. Pharmacogn. Rev. 2013, 7, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hou, T.T.; Xin, H.L.; Zhang, Q.Y.; Zheng, H.C.; Rahman, K.; Qin, L.P. Estrogen-Like activity of volatile components from Vitex rotundifolia L. Indian J. Med. Res. 2007, 126, 68–72. [Google Scholar] [PubMed]
- Ono, M.; Yamamoto, M.; Yanaka, T.; Ito, Y.; Nohara, T. Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem. Pharm. Bull. 2001, 49, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Yanaka, T.; Yamamoto, M.; Ito, Y.; Nohara, T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J. Nat. Prod. 2002, 65, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.G.; Kang, T.H.; Lee, S.J.; Kim, N.Y.; Kim, Y.C.; Sohn, D.H.; Lee, B.H. Polymethoxyflavonoids from Vitex rotundifolia inhibit proliferation by inducing apoptosis in human myeloid leukemia cells. Food Chem. Toxicol. 2000, 38, 861–865. [Google Scholar] [CrossRef]
- Paliany, A.S.; Sivasothy, Y.; Awang, K.; Rizman-Idid, M.; Alias, S.A. Marine derived fungi of peninsular Malaysia—A biochemical perspective. Chiang Mai J. Sci. 2014, 41, 894–909. [Google Scholar]
- Khan, S.A.; Hamayun, M.; Khan, A.L.; Lee, I.; Shinwari, Z.K.; Kim, J. Isolation of plant growth promoting endophytic fungi from dicots inhabiting coastal sand dunes of Korea. Pak. J. Bot. 2012, 44, 1453–1460. [Google Scholar]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Müller, W.E.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.; Lin, W.H.; et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- Abbas, H.K.; Riley, R.T. The presence and phytotoxicity of fumonisins and aal-toxin in Alternaria alternata. Toxicon 1996, 34, 133–136. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine-derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; Di Toppi, L.S.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Stacpoole, P.W. The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell 2012, 11, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.L.; Hu, X.; Tam, K.Y. Targeting tumor metabolism for cancer treatment: Is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int. J. Biol. Sci. 2015, 11, 1390–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Lunt, S.Y.; Van der Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 2, 441–464. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Jeon, J.H.; Min, B.K.; Ha, C.M.; Thoudam, T.; Park, B.Y.; Lee, I.K. Role of the pyruvate dehydrogenase complex in metabolic remodeling: Differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab. J. 2018, 42, 270–281. [Google Scholar] [CrossRef]
- Fernandez-Sada, E.; Silva-Platas, C.; Villegas, C.A.; Rivero, S.L.; Willis, B.C.; Garcia, N.; Garza, J.R.; Oropeza-Almazan, Y.; Valverde, C.A.; Mazzocchi, G.; et al. Cardiac responses to β-adrenoceptor stimulation is partly dependent on mitochondrial calcium uniporter activity. Br. J. Pharmacol. 2014, 171, 4207–4221. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, R.; Pirinen, E.; Lamperti, C.; Marchet, S.; Sauve, A.A.; Li, W.; Leoni, V.; Schon, E.A.; Dantzer, F.; Auwerx, J.; et al. NAD+-dependent activation of sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014, 19, 1042–1049. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Xiong, X.; Harris, R.A.; White, M.F.; Dong, X.C. Genetic inactivation of pyruvate dehydrogenase kinases improves hepatic insulin resistance induced diabetes. PLoS ONE 2013, 8, e71997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, Y.; Jeong, J.Y.; Jeoung, N.H.; Jeon, J.H.; Park, B.Y.; Kang, H.J.; Ha, C.M.; Choi, Y.K.; Lee, S.J.; Ham, H.J.; et al. Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes 2016, 65, 2876–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tso, S.C.; Qi, X.; Gui, W.J.; Wu, C.Y.; Chuang, J.L.; Wernstedt-Asterholm, I.; Morlock, L.K.; Owens, K.R.; Scherer, P.E.; Williams, N.S.; et al. Structure-Guided development of specific pyruvate dehydrogenase kinase inhibitors targeting the ATP-binding pocket. J. Biol. Chem. 2014, 289, 4432–4443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, F.; Chen, G.D.; He, J.W.; Li, X.X.; Sun, X.; Guo, L.D.; Li, Y.; Gao, H. Xinshengin, the first altenusin with tetracyclic skeleton core from Phialophora spp. Tetrahedron Lett. 2013, 54, 4551–4554. [Google Scholar] [CrossRef]
- Onocha, P.A.; Okorie, D.A.; Connolly, J.D.; Roycroft, D.S. Monoterpene diol, iridoid glucoside and dibenzo-α-pyrone from Anthocleista djalonensis. Phytochemistry 1995, 40, 1183–1189. [Google Scholar] [CrossRef]
- Lou, J.; Yu, R.; Wang, X.; Mao, Z.; Fu, L.; Liu, Y.; Zhou, L. Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivities. Braz. J. Microbiol. 2016, 47, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Nemecek, G.; Cudaj, J.; Podlech, J. Revision of the structure and total synthesis of altenuisol. Eur. J. Org. Chem. 2012, 3863–3870. [Google Scholar] [CrossRef]
- He, J.W.; Chen, G.D.; Gao, H.; Yang, F.; Li, X.X.; Peng, T.; Guo, L.D.; Yao, X.S. Heptaketides with antiviral activity from three endolichenic fungal strains Nigrospora sp., Alternaria sp. and Phialophora sp. Fitoterapia 2012, 83, 1087–1091. [Google Scholar] [CrossRef]
- Jiao, P.; Gloer, J.B.; Campbell, J.; Shearer, C.A. Altenuene derivatives from an unidentified freshwater fungus in the family tubeufiaceae. J. Nat. Prod. 2006, 69, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.X.; Bao, L.; Yang, X.L.; Guo, H.; Yang, R.N.; Ren, B.; Zhang, L.X.; Dai, H.Q.; Guo, L.D.; Liu, H.W. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia 2012, 83, 209–214. [Google Scholar] [CrossRef]
- Stack, M.E.; Mazzola, E.P.; Page, S.W.; Pohland, A.E.; Highet, R.J.; Tempesta, M.S.; Corley, D.G. Mutagenic perylenequinone metabolites of Alternaria alternata: Altertoxins I, II, and III. J. Nat. Prod. 1986, 49, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.M.; Schatzle, M.A.; Ludeke, S.; Muller, M. Unprecedented role of hydronaphthoquinone tautomers in biosynthesis. Angew. Chem. Int. Ed. 2004, 53, 9806–9811. [Google Scholar] [CrossRef] [PubMed]
- Gu, W. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana. World J. Microbiol. Biotechnol. 2009, 25, 1677–1683. [Google Scholar] [CrossRef]
- Bensassi, F.; Gallerne, C.; Sharaf El Dein, O.; Hajlaoui, M.R.; Bacha, H.; Lemaire, C. Cell death induced by the alternaria mycotoxin alternariol. Toxicol. In Vitro 2012, 26, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Schreck, I.; Deigendesch, U.; Burkhardt, B.; Marko, D.; Weiss, C. The alternaria mycotoxins alternariol and alternariol methyl ether induce cytochrome P450 1A1 and apoptosis in murine hepatoma cells dependent on the aryl hydrocarbon receptor. Arch. Toxicol. 2012, 86, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-C.; Zhang, Y.-M.; Hu, L.; Ma, Y.-T.; Gao, J.-M. Cytotoxic metabolites produced by Alternaria No.28, an endophytic fungus isolated from Ginkgo biloba. Nat. Prod. Commun. 2009, 4, 1473–1476. [Google Scholar]
- Zajkowski, P.; Grabarkiewicz-Szcesna, J.; Schmidt, R. Toxicity of mycotoxins produced by four Alternaria species to Artemia salina larvae. Mycotoxin Res. 1991, 7, 11–15. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dallin, S. Toxicity of the Alternaria spp. metabolites, tenuazonic acid, alternariol, altertoxin-i, and alternariol monomethyl ether to brine shrimp (Artemia salina L.) larvae. J. Sci. Food Agric. 1994, 66, 493–496. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Moore, G.W.; Kornhauser, D.M.N. Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N. Engl. J. Med. 1978, 298, 526–530. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |

| Position | δC | δH (J in Hz) | Position | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 165.5 | 9 | 125.8 | 7.17, s | |
| 2 | 99.6 | 10 | 196.5 | ||
| 3 | 163.8 | 11 | 87.5 | ||
| 4 | 105.0 | 6.81, d (2.0) | 11-OH | 7.25, s | |
| 5 | 166.0 | 12 | 88.8 | ||
| 5-OCH3 | 56.4 | 3.92, s | 13 | 24.3 | 1.51, s |
| 6 | 106.0 | 7.23, d (2.0) | 14 | 168.0 | |
| 7 | 131.5 | 14-OCH3 | 53.0 | 3.61, s | |
| 8 | 163.5 | 3-OH | 11.12, s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Li, W.; Bang, S.; Lee, S.J.; Kang, N.-y.; Kim, S.; Kim, T.I.; Go, Y.; Shim, S.H. Secondary Metabolites of The Endophytic Fungus Alternaria alternata JS0515 Isolated from Vitex rotundifolia and Their Effects on Pyruvate Dehydrogenase Activity. Molecules 2019, 24, 4450. https://doi.org/10.3390/molecules24244450
Lee C, Li W, Bang S, Lee SJ, Kang N-y, Kim S, Kim TI, Go Y, Shim SH. Secondary Metabolites of The Endophytic Fungus Alternaria alternata JS0515 Isolated from Vitex rotundifolia and Their Effects on Pyruvate Dehydrogenase Activity. Molecules. 2019; 24(24):4450. https://doi.org/10.3390/molecules24244450
Chicago/Turabian StyleLee, Changyeol, Wei Li, Sunghee Bang, Sun Joo Lee, Nam-young Kang, Soonok Kim, Tae In Kim, Younghoon Go, and Sang Hee Shim. 2019. "Secondary Metabolites of The Endophytic Fungus Alternaria alternata JS0515 Isolated from Vitex rotundifolia and Their Effects on Pyruvate Dehydrogenase Activity" Molecules 24, no. 24: 4450. https://doi.org/10.3390/molecules24244450






