Abstract
Thirty phenolic ether derivatives of scopoletin modified at the 7-hydroxy position were synthesized, and their structures were confirmed by IR, 1H-NMR, 13C-NMR, MS and elemental analysis. Preliminary acaricidal activities of these compounds against female adults of Tetranychus cinnabarinus (Boisduval) were evaluated using the slide-dip method. The results indicated that some of these compounds exhibit more pronounced acaricidal activity than scopoletin, especially compounds 32, 20, 28, 27 and 8 which exhibited about 8.41-, 7.32-, 7.23-, 6.76-, and 6.65-fold higher acaricidal potency. Compound 32 possessed the the most promising acaricidal activity and exhibited about 1.45-fold higher acaricidal potency against T. cinnabarinus than propargite. Statistically significant 2D-QSAR model supports the observed acaricidal activities and reveals that polarizability (HATS5p) was the most important parameter controlling bioactivity. 3D-QSAR (CoMFA: q2 = 0.802, r2 = 0.993; CoMSIA: q2 = 0.735, r2 = 0.965) results show that bulky substituents at R4, R1, R2 and R5 (C6, C3, C4, and C7) positions, electron positive groups at R5 (C7) position, hydrophobic groups at R1 (C3) and R2 (C4), H-bond donors groups at R1 (C3) and R4 (C6) will increase their acaricidal activity, which provide a good insight into the molecular features relevant to the acaricidal activity for further designing novel acaricidal agents. Molecular docking demonstrates that these selected derivatives display different bide modes with TcPMCA1 from lead compound and they interact with more key amino acid residues than scopoletin. In silico ADME properties of scopoletin and its phenolic ether derivatives were also analyzed and showed potential to develop as good acaricidal candidates.
1. Introduction
The carmine spider mite, Tetranychus cinnabarinus (Boisduval), is considered as one of the most economically important arthropod pests [1]. This mite has been reported to infest over 100 crops or plants grown in the field or greenhouse worldwide, especially cotton, beans, eggplants, tomatoes, peppers, cucurbits and strawberries and so on [2,3,4]. Spider mites usually feed through a piercing-sucking process to remove cellular contents, resulting in reduction of photosynthesis and transpiration rates in plants [5,6]. The plants slightly infested by spider mite display discoloration of their leaves and defoliation, bud and fruit dropping and reductions in fruit yield and quality; serious plants infestations by this mite will cause whole plant death [7]. It is recognized as one of the most difficult mites to control mainly due to its small size, high reproductive potential, extremely short life cycle, and strong adaptability and ability to develop resistance [8,9]. The genetic system of spider mites is known as arrhenotoky, wherein unfertilized haploid eggs develop into males and fertilized eggs develop into females [6]. This type of genetic system is highly vulnerable to mutations, conferring resistance to acaricides [10]. In addition, for a long time, this pest mite was controlled mainly through frequent applications of synthetic chemical acaricides, which have resulted in mite resistance to almost all major classes of acaricides throughout the world as well as environmental problems [11,12,13,14,15]. Therefore, it is necessary to develop novel, powerful, and environmentally-friendly acaricides from natural products, which will be used as an alternative agent to control this pest mite.
Scopoletin is an important secondary metabolite found in many plant species, such as Erycibe obtusifolia Benth [16], Aster tataricus [17], Foeniculum vulgare [18], Artemisia annua L. [19], Sinomonium acutum [20], and Melia azedarach L. fruits [21]. Scopoletin is classified as a coumarin and chemically known as 7-hydroxy-6-methoxy-2H-chromen-2-one [22]. Studies have shown that scopoletin has a wide spectrum of biological activities, such as pronounced acaricidal [23,24], anti-inflammatory [25,26], antitumoral [27], antioxidative [20], hepatoprotective [28], insecticidal [29], antifungal [30], and alleopathic properties [31]. Based on its pronounced acaricidal activities, our research group further investigated the mechanism of action and found that Ca2+-ATPase, which is vital in nervous signal conduction [32,33,34], was inhibited [35] and TcPMCA1 from Ca2+-ATPase was significantly upregulated after T. cinnabarlnus was exposed to scopoletin, and molecular docking also showed that scopoletin inserts into the binding cavity and interacts with TcPMCA1 protein through the driving forces of hydrogen bonds [36]. However, its acaricidal activity remain lower than that of some registered synthetic chemical acaricides, such as pyridaben. To date, few studies have attempted to improve the acaricidal effects of scopoletin by modifying its structure.
Quantitative structure-activity relationship (QSAR) and molecular docking are two important computational approaches, which have been considered as effective facilitating tools in drug design and discovery [37,38]. Quantitative structure-activity relationship (QSAR) is a method that correlates chemical structure of the compound with its biological activity [39,40,41]. QSAR has also been widely used to provide useful information for the design and discovery of insecticidal and acaricidal agents [42,43]. Molecular docking is a computational method to identify targets or find possible binding modes of the compound against its biological target, and has been successfully used to investigate binding modes of many classes of pesticides [44,45].
Therefore, our interest now focused on the modification of scopoletin to increase its acaricidal potency by using a molecular hybridization method. A series of scopoletin phenolic ether derivatives were designed and synthesized. All the target compounds were characterized by IR, 1H-NMR, 13C-NMR, MS, and elemental analysis, and their acaricidal activities against female adults of T. cinnabarlnus were evaluated. QSAR and molecular docking were also performed to provide useful structure-activity relationship information for the discovery of novel acaricidal agents and insights into the important interaction of compounds and TcPMCA1. An in silico study of scopoletin and its synthetic phenolic ether derivatives was performed to predict their ADME properties.
2. Results and Discussion
2.1. Chemistry
The synthesis of scopoletin (4) is outlined in Scheme 1. The first two reaction steps of synthesizing 4 (scopoletin) followed literature methods [22,46], and the last step was altered by adding the 7-hydroxy-6-methoxy-2-oxo-2H-chromene-3-carboxylic acid (3) to pyridine and ethylene glycol (1:1.1) and heating under microwave irradiation for 50 min to afford scopoletin (4).
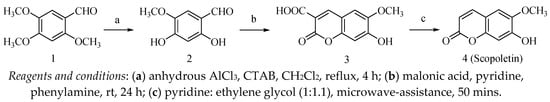
Scheme 1.
Synthesis of scopoletin.
The synthesis of target compounds 8–37 is outlined in Scheme 2. Target compounds 8–25 were obtained from scopoletin through a one-step reaction with alkyl or aromatic halides. To increase the electronegativity of the oxygen atom of the hydroxyl from scopoletin, we first added scopoletin and K2CO3 to acetone and stirred at reflux; subsequently alkyl or aromatic halides were added into the mixed reaction solution to react, leading to the acceptable yields of the derivatives.
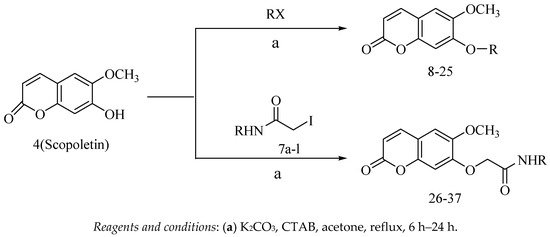
Scheme 2.
Synthesis of scopoletin phenolic ether derivatives (8–37).
Target compounds 26–37 were obtained through a three-step reaction. The intermediates 6a–l were synthesized through the reaction of 2-chloroacetyl chloride with an alkylamine or substituted benzylamine. To quickly remove the by-product hydrochloric acid from this reaction, we used triethylamine as acid-binding agent, which was added before adding 2-chloroacetyl chloride into the reaction mixture. Chlorinated intermediates 6a–l were converted into iodine-substituted intermediates 7a–l (Scheme 3) to obtain high yields of the target compounds. Finally, the target compounds 26–37 were synthesized by reacting scopoletin with intermediates 7a–l in acetone. All of the target compounds provided satisfactory analytical and spectroscopic data, which were consistent with their depicted structures.
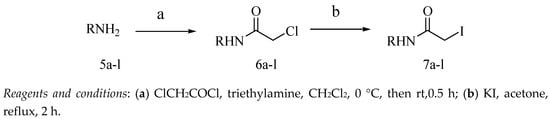
Scheme 3.
Synthesis of iodoacetamide derivatives.
2.2. Acaricidal Activity
As shown in Table 1, all the tested compounds exhibited varying degrees of acaricidal potency against female adults of T. cinnabarinus after treatment for 48 h, LC50 (mmol/L) and χ2 values of all tested compounds are less than 6.6 and 5.6, respectively, pLC50 (mol/L) and P values of all tested compounds are more than 2.0 and 0.1, respectively. Except for compound 15, the rest of the target compounds exhibited more pronounced acaricidal activities against T. cinnabarinus than scopoletin. In particular, compounds 32, 20, 28, 27 and 8 exhibited about 8.41-, 7.32-, 7.23-, 6.76-, and 6.65-fold higher acaricidal potency than the lead compound. Compound 32 possessed the the most promising acaricidal activity and exhibited about 1.45-fold higher acaricidal potency against T. cinnabarinus than propargite.

Table 1.
Contact activity of scopoletin and its phenolic ether derivatives (8–37) against female adults of T. cinnabarinus (48 h).
The acaricidal activity of compounds 8–13 decreased as the carbon chain length of the substituent groups increased. Compounds 14–17 with a naphthenic base displayed lower acaricidal activity. When the hydrogen of a hydroxyl or amino from compounds 18–25 and 32–37 was substituted by a substituted phenyl group, different acaricidal potency was shown. This is due to the types, quantity and position of substituents on the benzene rings of these compounds. However, all of them well followed the Topliss tree rule [47]. Compound 23 with a para-chlorinated phenyl (pLC50 = 2.3759) exhibited low potency against T. cinnabarinus compared with compound 18 with a simple phenyl (pLC50 = 2.5922), therefore, both compound 19 with para-methylphenyl (pLC50 = 2.4954) and compound 22 with 3,4 dichlophenyl (pLC50 = 2.7821) don’t show excellent potency against T. cinnabarinus. Compound 33 with a para-chlorinated phenyl containing an amide group (pLC50 = 3.0716) exhibited equivalent potency against T. cinnabarinus compared with compound 31 with a phenyl-containing amide group (pLC50 = 2.9452), compound 35 with a para-methylphenyl-containing amide group exhibited low potency against T. cinnabarinus (pLC50 = 2.7510) compared with compound 33, therefore, the compound 32 with a 3-chlorophenyl-containing amide group (pLC50 = 3.1835) show higher acaricidal potency. Both compound 34 with a 3,4-dichlorophenyl-containing amide group (pLC50 = 3.0165) and compound 36 with a para-methoxyphenyl-containing amide group (pLC50 = 2.8193) don’t show good potency against T. cinnabarinus.
The amino hydrogens of compounds 26–31 were substituted by alkyl groups, which also followed the Topliss tree rule [47]. Compound 29 with isopropyl (pLC50 = 2.5165) show equivalent potency against T. cinnabarinus compared with compound 26 with methyl (pLC50 = 2.5950), and compound 27 with an ethyl moiety shows prominent (pLC50 = 3.0888) acaricidal potency.
In addition, the acaricidal activity of the compounds 32–34 containing amide groups with benzene rings substituted by electron-withdrawing groups (3-chloro-, 4-chloro-, and 3,4-dichloro-) was higher than that of benzene rings substituted by electron-donating groups (4-methyl-, 4-methoxy- and 4-tert-butyl-) (compounds 35–37).
The 48 h LC50 value of scopoletin in the current study was different from our previous reports [23], which may be attributed to the differences in scopoletin purity, the pesticide adjuvants, and the solvents used to prepare the tested compounds.
Compound 8 was used as typical representative of all target compounds to evaluate acaricidal activity against eggs, larval, and nymphal of T. cinnabarinus, basing on its higher acaricidal potency against female adults. As shown in Table 2, compound 8 exhibits excellent acaricidal potency against larva, low activity against nymphs, and no ovicidal activity. The different acaricidal potency maybe due to different expression of the possible target gene TcPMCA1 at different stages of T. cinnabarinus [36].

Table 2.
Acaricidal activity of compound 8 against eggs, larvae, and nymphs of T. cinnabarinus (48 h).
2.3. QSAR Analysis
2.3.1. 2D-QSAR Analysis
The selected descriptors, their correlations and their values of the investigated compounds are provided in Table 3, Table 4 and Table 5, respectively. The best performing 2D-QSAR models was successfully constructed as shown in Equation (1):
pLC50 = 4.243 (± 0.704) − 1.045 (± 0.218) R8e + 12.920 (± 1.921) HATS5p + 0.313 (± 0.062) Depressant − 80 − 1.351 (±0.385) MATS6e − 1.274 (± 0.401) HNar
N = 25, n =5, R = 0.935, R2train = 0.875, R2adjusted = 0.842, RMSEtrain = 0.1095, F = 26.527 > F0.005(5,25) = 4.43 (the cut off value of F distribution)
R LOO = 0.876, R2 LOO = 0.768, R2 LOO adjusted = 0.758, and RMSE LOO = 0.1299, F = 75.998, R2pred = 0.583

Table 3.
Selected descriptors of multiple linear regression.

Table 4.
The correlation matrix of descriptors.

Table 5.
Values of significant molecular descriptors, experimental and predicted acaricidal activities (pLC50, mol/L) from 2D-QSAR of the compounds 8–37.
The R2train value of this model reveals that it can explain 87.5% of the variances in activity. Root mean square error (RMSEtrain = 0.1095) is also a measurable value for the attained model together with the Fisher test value (F = 26.527) which reflects the ratio of the variance explained by the model and the variance due to their errors. A high value of F-test compared with the RMSE is a validation of the model.
To determine whether multicollinearity existed among the descriptors in the models or not, a variable inflation factor (VIF) (VIF = 1/(1 − Rj2), where Rj2 represents the multiple correlation coefficient of one descriptor’s effect on the remaining molecular descriptors) was calculated for each variable in the regression equation [48]. If VIF ranges from 1.0 to 5.0, the linked equation is suitable [49]. As shown in Table 3, the VIF of all descriptors were smaller than 2, indicating that the generated model possessed statistical significance and good stability. Table 4 gives the correlation matrix of the selected descriptors. From this table, it can be seen that the linear correlation coefficient value for each pair of descriptors was smaller than 0.6, suggesting that the selected descriptors were independent, meeting the important criterion for the model selections [50].
The reliability and statistical relevance of the attained BMLR-QSAR model is examined by internal and external validation procedures. Experimental and predicted activities (pLC50, mol/L) values of the compounds are shown in Table 5 and Figure 1. The residual values obtained by calculating the difference between the predicted and experimental pLC50 are below 0.35 logarithmic units for all the compounds.
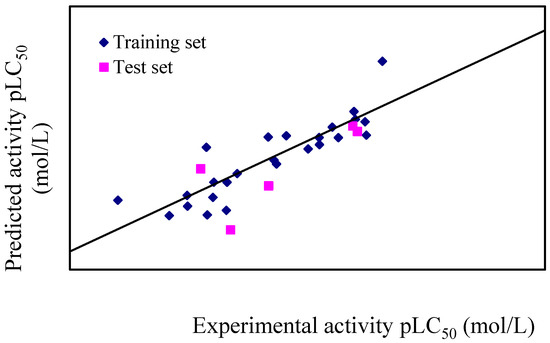
Figure 1.
Plot of experimental versus predicted pLC50 (mol/L) values of training sets and test sets against female T. cinnabarinus.
Internal validation is applied by the SPSS technique employing Leave One Out (LOO), which involves developing a number of models with one example omitted at a time. The observed correlations due to the internal validation techniques are R2LOO = 0.768. The R2LOO value was bigger than 0.5, indicating that the developed model had good stability and predictive ability [48].
The synthesized thirty target compounds were randomly divided into a 25-molecule training set with LC50 values range from 0.655 to 6.588 mmol/L and a 5-molecule (11, 16, 19, 27, and 33) test set with LC50 values range from 0.815 to 3.196 mmol/L were used as an external test set for validating the attained QSAR models. The predicted/estimated acaricidal properties of the test set compounds are close to their experimentally observed values preserving their potencies. In addition, the value of R2pred = 0.583 for the external prediction was an acceptable result, which conformed that the generated MLR model was useful for meaningful predictions.
The QSAR model indicated that the descriptors representing polarizability (HATS5p) is main property governing acaricidal active agent of the scopoletin phenolic ether derivatives as shown by its high regression coefficient values of 12.920 (Equation (1)). The QSAR model demonstrated that high values of HATS5p, and Depressant-80, but low value of R8e, MATS6e, and HNar are required for potent activity of the compounds. Among these compounds 8–25, compounds 8 and 9 substituted by methyl and ethyl possessed high polarizability (HATS5p: 0.118 and 0.146) and low electronegativity (R8e: 0.384 and 0.706) exhibited high acaricidal potency. Among compounds 25–37, compounds 28 and 32 containing an amide group substituted by n-propyl and 3-Cl-benzyl possessed high polarizability (HATS5p: 0.115 and 0.133) and low electronegativity (R8e: 0.609 and 0.567; MATS6e: 0.037 and −0.063) and also displayed high acaricidal potency.
2.3.2. 3D-QSAR Analysis
Table 6 shows the PLS results of the CoMFA and CoMSIA models. The results showed that the optimal CoMFA model yielded a cross-validated q2 = 0.802 with an optimal number of principal components (ONC) of 6, non-cross-validated R2 of 0.993, SEE = 0.029 and F value of 422.047. The contribution of steric and electrostatic fields is 70.8% and 29.2%, respectively. The best CoMSIA model yielded a q2 of 0.735 with an ONC of 6, non-cross-validated R2 of 0.965, SEE = 0.059 and F value of 83.553. The contribution of steric, electrostatic, hydrophobic, and hydrogen-bond acceptor are 21.5%, 28.5%, 44.9%, and 5.0%, respectively. Based on these field contributions, the steric field is the most important field in the CoMFA model, whereas the hydrophobic field is the most important field in the CoMSIA model. All the parameters in the Table 6 indicate that the CoMFA and CoMSIA models are robust and stable.

Table 6.
Summary of the results obtained from CoMFA and CoMSIA analyses.
The plot of experimental versus predicted acaricidal activities for CoMFA and CoMSIA models are shown in Table 7, and Figure 2 and Figure 3. The residual values obtained by calculating the difference between the predicted and experimental pLC50 are below 0.3 logarithmic unit for all the compounds. In addition, the values of R2pred = 0.999 (CoMFA) and R2pred = 0.787 (CoMSIA) for the external prediction were acceptable results. The CoMFA R2pred is higher than its R2, which indicated CoMFA model higher predictive ability. The prediced pLC50 values of five test compounds by CoMFA model are very close to their experimental pLC50 values, and their residuals are less than 0.008 logarithmic unit. These results indicate that the CoMFA and CoMSIA models are predictive.

Table 7.
Experimental and predicted acaricidal activities (pLC50, mol/L) from 3D-QSAR of the compounds 8–37.
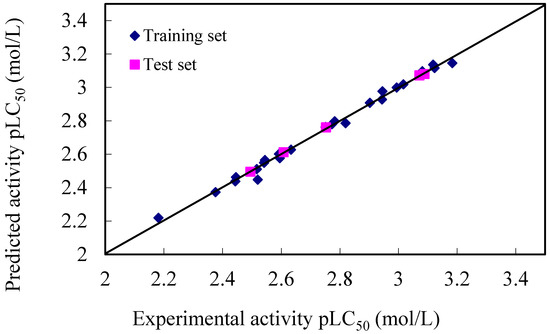
Figure 2.
The plot of experimental versus calculated pLC50 values from CoMFA analyses for the training and test set compounds.
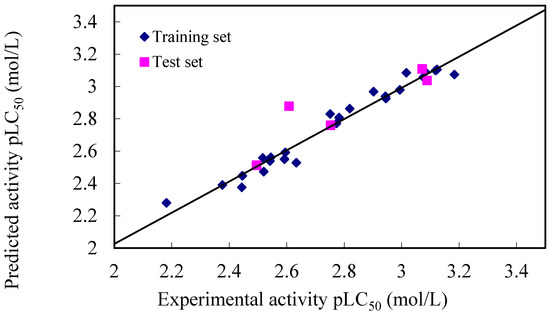
Figure 3.
The plot of experimental versus calculated pLC50 values from CoMSIA analyses for the training and test set compounds.
Core structure of the studied scopoletin phenolic ether derivatives were shown in Figure 4A, the compound 8 was employed as the template molecule for the analysis of contour maps (Figure 4B).
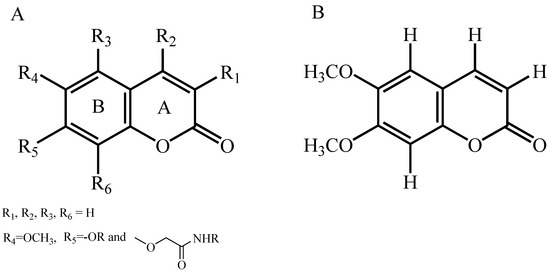
Figure 4.
(A) The skeleton structure of the studied scopoletin phenolic ether derivatives and (B) the chemical structure of compound 8.
The CoMFA steric and electrostatic contour maps are shown in Figure 5 with compound 8. Those contours depict default contribution levels. In the CoMFA steric field shown in Figure 5A, A large-sized and two medium-sized green contour near R4, R1, R2 and R5 (C6, C3, C4, and C7) indicate that bulky substituents were preferred here. It can be explained that the most of synthesized 7-position scopoletin phenolic ether derivatives have higher acaricidal activity than scopoletin. For CoMFA electrostatic map (Figure 5B), there is one blue contour around the R5 (C7) position, which can be explain the fact that compound 8 with the smallest electronegative OCH3 groups possesses higher acaricidal activity among the synthesized target compounds.
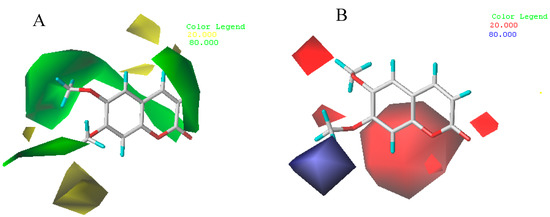
Figure 5.
CoMFA STDEV*COEFF contour maps around the compound 8. (A) Steric fields: green contours indicate regions where bulky groups increase activity, while yellow contours indicate regions where bulky groups decrease activity; and (B) electrostatic fields: blue contours indicate regions where electron positive groups increase activity, while red contours indicate regions where electron negative groups increase activity. Compound 8 is displayed as a reference.
CoMSIA steric, electrostatic, hydrophobic, hydrogen-bond acceptor field contour maps are shown in Figure 6 with compound 8 as an example. Those contours also depict the default contribution levels. Since the steric and electrostatic contour are very similar with that of CoMFA, only hydrophobic, hydrogen-bond acceptor will be described as follows: in the hydrophobic contour map (Figure 6C), a large-sized yellow contour near R1 (C3) and R2 (C4) indicates that introducing hydrophobic groups to that position could increase the acaricidal activity of the molecule. A large-sized white contour near R6 (C8) suggests that hydrophilic substitutes preferentially localize at these positions. A medium-sized white contour were found surrounding the R5 (C7) which indicates that introducing hydrophilic groups to this position could improve the acaricidal activity. Therefore, the synthesized target compouds (e.g., 27, 28 and 32) containing amide groups show higher acaricidal activity.
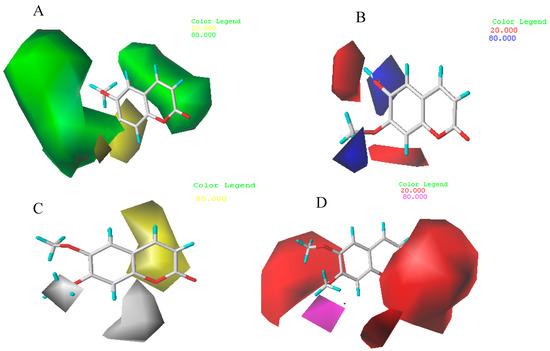
Figure 6.
CoMSIA STDEV*COEFF contour maps around the compound 8. (A) Steric fields: green contours and yellow contours show regions where an increase in bulky groups will increase and decrease activity, respectively; (B) electrostatic fields: blue contours and red contours show regions where an increase in electron positive groups and electron negative groups will increase activity, respectively; (C) hydrophobic fields: yellow contours and white contours show regions where an increase in lipophilicity and hydrophilicity will increase activity, respectively; (D) H-bond acceptor contour map: magenta contour and red contour show regions where an increase in hydrogen-bond acceptor groups and hydrogen-bond donor groups will increase activity.
In H-bond acceptor contour maps (Figure 6D), two large-sized red contours near R1 (C3) and R4 (C6) indicate that introducing H-bond donors groups at those positions could increase the acaricidal activity. A medium–sized magenta contour near R5 (C7) suggests that H-bond acceptor groups at this position are favorable, and will increase the molecular activity. For example, several compounds (e.g., 8, 9 and 12) with H-bond acceptor groups display higher acaricidal activity.
In this research, only R5 (C7)-position was be modified to investigate acaricidal activity, and the contours maps of CoMFA and CoMSIA-derived models suggest that some favored group introduced to other position of scopoletin could improve acaricidal activity, which need to be further study.
2.4. Molecular Docking
Some tested compounds exhibit higher acaricidal activity than scopoletin, which prompted us to performed molecular docking study to understand the ligand-the target protein Ca2+-ATPase interactions in detail. Scopoletin and the synthesized derivatives 8, 9, 12, 20 and 28 possessing the higher acaricidal activity were selected for the docking study.
Docking results of scopoletin and its derivatives 8, 9, 12, 20 and 28 binding to TcPMCA1 are listed in Table 8. Scopoletin and selected compound show low binding energies of much less than 5.0 kcal/mol, which can be generally considered as specific ligands of TcPMCA1. Figure 7 shows the binding modes and orientations of scopoletin and its derivatives to TcPMCA1. The two dimensional interaction diagrams of scopoletin and its derivatives to TcPMCA1 are shown in Figure 8. Scopoletin exhibits different binding poses compared to its derivatives. Five key amino acids (ASP222, ASP213, GLU220, VAL224, and THR218) in the binding pocket interact with scopoletin via hydrogen bonding and hydrophobic interaction. The H atom of hydroxyl at the 7-position in ring B forms a conventional hydrogen bond (-H…OC-, 2.05 Å) with ASP222, the H atom of methoxy group at the 6-position in ring B forms two nonconventional hydrogen bond (-H…OC-, 2.81 Å; -H…OC-, 3.33 Å) with ASP213, and GLU220 respectively. The benzene and furan rings of scopoletin formed two pi–alkyl (4.07 Å and 4.88 Å) interactions with the VAL224, and furan ring of scopoletin formed a pi–sigma (2.23 Å) interaction with the THR218. In addition, acid residues GLU214, SER215, HIS223, SER221, and ARG781 in the binding pocket interact with scopoletin via Van der Waals interactions. Derivatives 8, 9, 12, and 20 display almost same binding mode, especially homologues 8, 9 and 12. The interactions of these compounds with TcPMCA1 are analyzed using compound 8 as an example. Six key amino acids (GLU214, ASP222, ASP213, GLY723, ASP724, and VAL224) in the binding pocket interact with compound 8 via hydrogen bonding and hydrophobic interaction. The O atom of carbonyl at the 2-position in ring A and O atom of furan ring form two conventional hydrogen bond (-O…NH-, 2.15 Å and -O…NH-, 1.97 Å) with GLU214, and ASP222, respectively. The H atom of the methoxy group at the 6,7-position in ring B and the O atom of carbonyl at the 2-position in ring A form three non-conventional hydrogen bonds (-H…OC-, 3.31, -H…OC-, 3.11, and -O…HC) with GLY723, ASP724, and ASP213, respectively. The furan and benzene rings of compound 8 form two pi–alkyl (4.53 Å and 5.47 Å) interactions with the VAL224. Compound 8 is also surrounded by SER215, HIS223, THR218, ARG781, ASN725, GLU220, and SER221 through Van der Waals interactions. There are few differences of specific biding poses between compounds 8, 9, 12 and 20. Compound 9 shows an analogous binding mode, except for some differences of binding bond length and two non-conventional hydrogen bonds only form at 6-position instead of the 6,7-positions, compared to compound 8. Compound 12 forms an alkyl–alkyl hydrophobic interaction at the 7-position with MET490 instead of a non-conventional hydrogen bond, compared to compound 8. Compound 20 forms pi–cation and pi–anion interactions with LYS481, ASP724, respectively, instead of non-conventional hydrogen bonds, compared to compound 8. Compound 28 with an amide group displays very different binding modes from compounds 8, 9, 12 and 20. Seven key amino acids (GLU214, VAL224, THR218, ASP213, GLY723, ARG781, and LEU217) in the binding pocket interact with compound 28 via hydrogen bonding and hydrophobic interactions. The O atom of the carbonyl at the 2-position in ring A, and the H atom of the amino group at the 7-position in ring B form three conventional hydrogen bonds (-O…NH-, 1.77 Å, -O…NH-, 2.96 Å, -H…OC-, 2.15 Å) with GLU214, VAL224, and THR218, respectively. The O atom of the carbonyl at the 2-position in ring A, the H atoms of the methoxy group at the 6-position, the O atom of the acylamino at the 7-position, the H atom of the methylene at the 7-position in ring B form three non-conventional hydrogen bonds (-O…HC-, 2.53 Å, -H…OC-, 3.16 Å, -O…CH-, 2.21 Å, and -H…OC-,3.53 Å) with ASP 213, GLY 723, ARG 781, and GLU 214, respectively. The furan and benzene rings of compound 28 form two pi–alkyl (3.78 Å and 4.45 Å) interactions with the VAL224. The N-propyl connected to the amide at the 7-position forms alkyl–alkyl interactions with the LEU217. Compound 28 interacts with ILE212, HIS223, ASP222, GLU220, ASN725, ASP724, PRO784, SER783, and SER782 through Van der Waals interactions. These selected derivatives display higher acaricidal activity that may be due to their different binding modes with TcPMCA1 from the lead compound and they interact with more key amino acid residues. Three selected homologues bind tighter with shortening of the side chain at the 7-position. The binding modes of scopoletin with TcPMCA1 in the current study were different from our previous reports [32], which may be attributed to our use of different software to find the active binding site interactions of TcPMCA1.

Table 8.
Binding energy and nonbonding interaction of scopoletin and its derivatives.
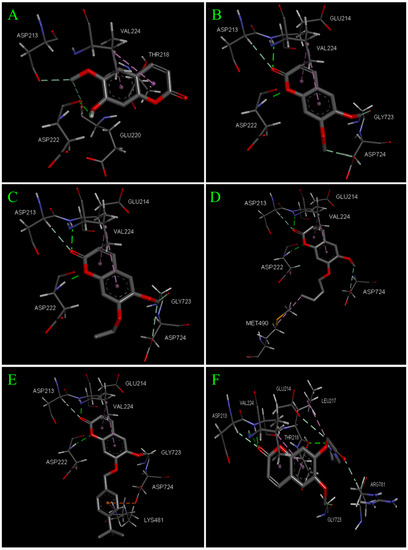
Figure 7.
The recognized binding modes and molecular interactions of the scopoletin (A) and compounds 8 (B), 9 (C), 12 (D), 20 (E), 28 (F) in the active site of TcPMCA1.
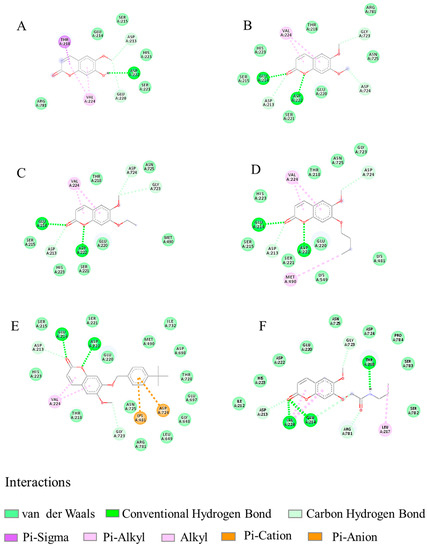
Figure 8.
Two dimensional interactions scheme of scopoletin (A) and compounds 8 (B), 9 (C), 12 (D), 20 (E), and 28 (F) to TcPMCA1.
2.5. ADME Study
An in silico study of scopoletin and its semisynthesed derivatives (compounds 8–37) was performed for prediction of ADME properties [51] (Table 9). From all these parameters, it can be observed that all tested compounds exhibited excellent % absorption (76.40–92.21%). It was also observed that all of these compounds followed Lipinski’s rule of five and its extensions well. Four typical Lipinski’s rule criteria are logP (octanol–water partition coefficient) ≤5, molecular weight ≤500, number of hydrogen bond acceptors ≤10 and number of hydrogen bond donors ≤5. Extension of Lipinski’s rule of five includes the following criteria: number of rotatable bonds ≤10, topological polar surface area ≤140 A2.

Table 9.
Evaluation parameters of Lipinski’s rule of five and its extensions from scopoletin and its phenolic ether derivatives (8–37).
3. Materials and Methods
3.1. General Information
Microwave-assisted synthesis was performed on a CW-2000 Ultrasonic Microwave Assisted Extractor (Xintuo Analytical Instruments Co., Ltd., Shanghai, China). Melting points were determined on a WRS-1B Digital Melting-Point Apparatus (Shanghai Shenguang Instrument Co., Ltd., Shanghai, China) and were uncorrected. IR spectra were obtained on a TENSOR 27 FT-IR spectrometer (Bruker Spectroscopic Instruments Co., Rheinstetten, Germany) using KBr pellets and values were presented in cm−1. 1H- and 13C-NMR spectra were recorded on an Avance III 400 NMR spectrometer (Bruker Spectroscopic Instruments Co., Rheinstetten, Germany) with CDCl3 or DMSO-d6 as solvent. Mass spectra were carried out with a GCMS-QP2010 Ultra instrument (Shimadzu Corporation, Kyoto, Japan). Elemental analyses were performed on a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany)Propargite 90.00% TC was provided by Qingdao Hansen Biologic Science Co., Ltd., (Qingdao, China), and all other chemicals and solvents were of analytical grade and used as purchased. Analytical thin-layer chromatography (TLC) was performed on a glass plate coated with silica gel GF-254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) and visualized under ZF-1 ultraviolet analyzer (Shanghai Gucun Electro-optical Instrument Factory, Shanghai, China) under UV light (254 nm). Column chromatography was performed on silica gel (200 to 300 mesh).
3.2. Chemistry
3.2.1. Procedure for the Synthesis of Scopoletin (4)
Preparation of 2,4-dihydroxy-5-methoxybenzaldehyde (2)
Following a literature method [22,46], aluminum (III) chloride (40 g, 0.30 mol) and CTAB (3.2 g, 8.8% mol) were added into dichloromethane (400 mL), and the reaction mixture was stirred at room temperature for 30 min, then a solution of 2,4,5-trimethoxybenzaldehyde (1, 20 g, 0.1 mol) in dichloromethane (100 mL) was added dropwise, then the mixture was refluxed for 4 h (the reaction progress was monitored by TLC with UV detection). The reaction mixture was cooled and poured onto 500 g of ice to which 100 mL of concentrated hydrochloric acid was added. The organic layer was separated and was washed with saturation salt solution, dried over anhydrous sodium sulfate, evaporated under reduced pressure to give 2,4-dihydroxy-5-methoxybenzaldehyde (2) as a light yellow solid, 60.84% yeild, m.p. 152–153 °C.
Preparation 7-hydroxy-6-methoxy-2-oxo-2H-chromene-3-carboxylic acid (3)
2,4-Dihydroxy-5-methoxybenzaldehyde (2, 9.49 g, 56 mmol), malonic acid (13.5 g, 130 mmol), and phenylamine (1 mL) were added into pyridine (30 mL), the resulting solution was stirred at room temperature for over 24 h and then acidified to pH 4 using dilute HCl. The precipitate was collected by suction filtration and followed by recrystallization from ethanol to give 7-Hydroxy-6-methoxy-2-oxo-2H-chromene-3-carboxylic acid (3) as a yellow solid, 80.01% yeild, m.p. 231–232 °C.
Preparation of 7-hydroxy-6-methoxy-2H-chromen-2-one (scopoletin, 4)
7-Hydroxy-6-methoxy-2-oxo-2H-chromene-3-carboxylic acid (3, 3.5 g, 14.8 mmol) was refluxed in ethylene glycol (17.6 mL) and pyridine (16 mL) for 50 min under microwave irradiation (115 W) and keep temperature above 110 °C. After cooling the reaction, the mixture was acidified to about pH 5 using a solution of diluted HCl 30 mL, then 7-hydroxy-6-methoxy-2H-chromen-2-one (scopoletin, 4) crystals were obtained after standing overnight, the filter liquor was extracted with CH2Cl2. The CH2Cl2 layers were pooled and washed with saturation sodium bicarbonate, and saturation salt solution, successively, dried over anhydrous sodium sulfate, evaporated under reduced pressure and followed by recrystallization from acetone to give the target product 4, 65.74% total yield, m.p. 201–202 °C.
3.2.2. General Procedure for the Synthesis of 8–13 and 18–25
K2CO3 (0.2073 g, 3 mmol) and CTAB (54.67 mg, 7.5% mmol) were added into a solution of scopoletin (4, 0.3843 g, 2 mmol) in acetone (30 mL), and the reaction mixture was stirred at reflux for 30 min. Then an alkyl or aromatic halide (3 mmol) was added into the mixture and maintained at reflux for 6–24 h (the reaction progress was monitored by TLC with UV detection). After cooling the reaction and filtration, the solvent was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate, washed with saturation sodium bicarbonate, and saturation salt solution, successively, dried over anhydrous sodium sulfate, evaporated under reduced pressure to give the target crude products. The crude products were purified by column chromatography using petroleum ether/ethyl acetate from 10:1 to 7:1 as the gradient eluent system to yield the products 8–13 and 18–25.
6,7-Dimethoxy-2H-chromen-2-one (8)
White needle-like crystals; 70.83% yield; m.p. 145.2–146.9 °C; IR νmax (KBr) cm−1: 3082, 2938, 2877, 1713, 1619, 1559, 1513, 1467, 1424, 1379, 1275, 1248, 1169, 1027, 880; 1H-NMR (DMSO-d6, δ ppm): 3.81 (s, 6H, 2 × OCH3), 6.30 (d, 1H, J = 8Hz, C3-H), 7.05 (s, 1H, C8-H), 7.25 (s, 1H, C5-H), 7.96 (d, 1H, J = 12 Hz, C4-H); 13C-NMR (DMSO-d6, δ ppm): 56.31, 70.56, 100.98, 109.39, 111.51, 112.99, 144.82, 146.36, 149.85, 152.32, 161.07; MS (m/z): [M]+ 206. Anal. Calcd. for C11H10O4: C, 64.08%; H, 4.89%. Found: C, 64.22%; H, 4.94%.
7-Ethoxy-6-methoxy-2H-chromen-2-one (9)
White granular crystals; 75.02% yield; m.p. 146.3–146.7 °C; IR νmax (KBr) cm−1: 3064, 2982, 2916, 2882, 2830, 1705, 1616, 1600, 1510, 1465, 1424, 1388, 1274, 1247, 1147, 1023, 877. 1H-NMR (DMSO-d6, δ ppm): 1.37 (t, 3H, J = 8 Hz, CH3), 3.81 (s, 3H, OCH3), 4.12 (q, 2H, J = 8 Hz, CH2), 6.30 (d, 1H, J = 12 Hz, C3-H), 7.04 (s, 1H, C8-H), 7.24 (s, 1H, C5-H), 7.95 (d, 1H, J = 8 Hz, C4-H) (Figure S1). 13C-NMR (DMSO-d6, δ ppm): 14.87, 56.24, 64.86, 100.95, 109.30, 111.51, 113.00, 144.81, 146.30, 149.84, 152.17, 161.06 (Figure S2). MS (m/z): [M]+ 220. Anal. Calcd. for C12H12O4: C, 65.45%; H, 5.49%. Found: C, 65.62%; H, 5.52%.
6-Methoxy-7-propoxy-2H-chromen-2-one (10)
White granular crystals; 78.08% yield; m.p. 96.0–96.7 °C, IR νmax (KBr) cm−1: 3084, 2967, 2939, 2877, 1705, 1619, 1559, 1513, 1467, 1424, 1386, 1275, 1248, 1150, 1027, 880. 1H-NMR (DMSO-d6, δ ppm): 0.99 (t, 3H, J = 8Hz, CH3), 1.73–1.82 (m, 2H, C′2-CH2), 3.82 (s, 3H, OCH3), 4.02 (t, 2H, J = 8 Hz, C′1-CH2), 6.30 (d, 1H, J = 12 Hz, C3-H), 7.04 (s, 1H, C8-H), 7.24 (s, 1H, C5-H), 7.95 (d, 1H, J = 8 Hz, C4-H) (Figure S3). 13C-NMR (DMSO-d6, δ ppm): 10.81, 22.26, 56.33, 70.57, 100.98, 109.42, 111.51, 112.98, 144.78, 146.38, 149.85, 152.34, 161.05 (Figure S4). MS (m/z): [M]+ 234. Anal. Calcd. for C13H14O4: C, 66.67%; H, 5.98%. Found: C, 66.99%; H, 6.08%.
7-Isopropoxy-6-methoxy-2H-chromen-2-one (11)
White needle crystals; 58.73% yield; m.p.108.4–108.5 °C; IR νmax (KBr) cm−1: 3098, 2979, 2939, 1706, 1613, 1560, 1513, 1464, 1424, 1383, 1269, 1245, 1148, 1025, 846. 1H-NMR (DMSO-d6, δ ppm): 1.31 (d, 6H, J = 8 Hz, CH3), 3.80 (s, 3H, OCH3), 4.71–4.80 (m, 1H, CH), 6.29 (d, 1H, J = 8 Hz, C3-H), 7.08 (s, 1H, C8-H), 7.25 (s, 1H, C5-H), 7.95 (d, 1H, J = 8 Hz, C4-H) (Figure S5). 13C-NMR (DMSO-d6, δ ppm): 22.03, 56.24, 71.15, 102.02, 109.63, 111.48, 112.96, 144.78, 146.91, 149.84, 151.03, 161.09 (Figure S6). MS (m/z): [M]+ 234. Anal. Calcd. for C13H14O4: C, 66.67%; H, 6.02%. Found: C, 66.82%; H, 6.21%.
7-Butoxy-6-methoxy-2H-chromen-2-one (12)
White granular crystals; 66.01% yield; m.p. 77.1–78.3 °C, IR νmax (KBr) cm−1: 3067, 2940, 2867, 1699, 1609, 1557, 1512, 1465, 1423, 1384, 1277, 1247, 1147, 1023, 873. 1H-NMR (DMSO-d6, δ ppm): 0.95 (t, 3H, J = 6 Hz, CH3), 1.40–1.49 (m, 2H, C′3-CH2), 1.70–1.79 (m, 2H, C′2-CH2), 3.81 (s, 3H, OCH3), 4.06 (t, 2H, J = 6 Hz, C′1-CH2), 6.29 (d, 1H, J = 8 Hz, C3-H), 7.05 (s, 1H, C8-H), 7.24 (s, 1H, C5-H), 7.95 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 14.11, 19.18, 30.93, 56.32, 68.85, 100.98, 109.40, 111.51, 112.97, 144.77, 146.38, 149.86, 152.37, 161.05. MS (m/z): [M]+ 248. Anal. Calcd. for C14H16O4: C, 67.73%; H, 6.50%. Found: C, 68.05%; H, 6.51%.
7-Isobutoxy-6-methoxy-2H-chromen-2-one (13)
White granular crystals; 27.85% yield; m.p. 84.1–85.3 °C; IR νmax (KBr) cm−1: 3096, 2965, 2921, 2876, 1714, 1613, 1561, 1514, 1457, 1425, 1386, 1266, 1248, 1143, 1013, 864. 1H-NMR (DMSO-d6, δ ppm): 1.00 (t, 3H, C′2-CH3), 1.44 (d, 3H, J = 8 Hz, C′1-CH3), 2.00–2.13 (m, 2H, CH2), 3.82 (s, 3H, OCH3), 3.83–3.94 (m, 1H, CH), 6.29 (d, 1H, J = 8 Hz, C3-H), 7.04 (s, 1H, C8-H), 7.25 (s, 1H, C5-H), 7.95 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 19.47, 28.02, 56.47, 75.21, 101.12, 109.61, 111.55, 113.02, 144.82, 146.45, 149.88, 152.45, 161.07. MS (m/z): [M]+ 248. Anal. Calcd. for C14H16O4: C, 67.73%; H, 6.50%. Found: C, 67.16%; H, 6.60%.
7-(Benzyloxy)-6-methoxy-2H-chromen-2-one (18)
White needle-like crystals; 72.41% yield; m.p. 124.6–125.6 °C; IR νmax (KBr) cm−1: 2959, 1715, 1611, 1561, 1510, 1462, 1427, 1379, 1274, 1247, 1145, 1027, 757. 1H-NMR (DMSO-d6, δ ppm): 3.93 (s, 3H, OCH3), 5.23 (s, 2H, CH2O), 6.27 (d, 1H, J = 8 Hz, C3-H), 6.87 (s, 1H, C8-H), 7.26 (s, 1H, C5-H), 7.33–7.45 (m, 5H, Ar-H), 7.61 (d, 1H, J = 8 Hz, C4-H) (Figure S9). 13C-NMR (DMSO-d6, δ ppm): 56.47, 71.13, 101.83, 108.36, 111.69, 113.59, 127.31, 128.37, 128.82, 135.63, 143.31, 146.77, 149.75, 151.83, 161.42 (Figure S10). MS (m/z): [M]+ 282. Anal. Calcd. for C17H14O4: C, 72.33%; H, 5.00%. Found: C, 72.07%; H, 4.98%.
6-Methoxy-7-(4-methylbenzyloxy)-2H-chromen-2-one (19)
White granular crystals; 80.17% yield; m.p. 126.1–127.0 °C; IR νmax (KBr) cm−1: 3075, 3011, 2944, 2929, 2861, 1705, 1616, 1563, 1518, 1460, 1425, 1392, 1279, 1249, 1145, 1020, 883. 1H-NMR (DMSO-d6, δ ppm): 2.31 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 5.15 (s, 2H, CH2O), 6.31 (d, 1H, J = 12Hz, C3-H), 7.15 (s, 1H, C8-H), 7.21 (s, 1H, C5-H), 7.24, 7.36 (dd, 4H, J = 8 Hz, Ar-H), 7.95 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 21.27, 56.32, 70.62, 101.60, 109.47, 111.78, 113.20, 128.69, 129.54, 133.55, 137.95, 144.77, 146.49, 149.65, 151.84, 161.02. MS (m/z): [M]+ 296. Anal. Calcd. for C18H16O4: C, 72.96%; H, 5.44%. Found: C, 72.53%; H, 5.26%.
7-(4-tert-Butylbenzyloxy)-6-methoxy-2H-chromen-2-one (20)
White sheet-like crystals; 83.23% yield; m.p. 149.4–149.7 °C; IR νmax (KBr) cm−1: 3082, 2948, 2862, 1720, 1610, 1560, 1508, 1460, 1424, 1387, 1272, 1242, 1141, 1015, 858. 1H NMR (DMSO-d6, δ ppm): 1.28 (s, 9H, 3 × CH3), 3.81 (s, 3H, OCH3), 5.16 (s, 2H, CH2O), 6.30 (d, 1H, J = 12 Hz, C3-H), 7.17 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.40, 7.43 (dd, 4H, J = 8 Hz, Ar-H), 7.95 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 31.56, 34.80, 56.35, 70.55, 101.56, 109.54, 111.79, 113.21, 125.75, 128.52, 133.60, 144.77, 146.51, 149.69, 151.15, 151.92, 161.00. MS (m/z): [M]+ 338. Anal. Calcd. for C21H22O4: C, 74.54%; H, 6.55%. Found: C, 75.00%; H, 6.54%.
7-(4-Nitrobenzyloxy)-6-methoxy-2H-chromen-2-one (21)
White powder; 60.42% yield; m.p. 122.3–122.9 °C; IR νmax (KBr) cm−1: 2946, 1735, 1603, 1562, 1519, 1465, 1427, 1347, 1284, 1251, 1174, 1010, 853. 1H-NMR (DMSO-d6, δ ppm): 3.95 (s, 3H, OCH3), 5.30 (s, 2H, CH2O), 6.31 (d, 1H, J = 12 Hz, C3-H), 6.92 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.63 (d, 1H, J = 4Hz, C4-H), 7.65 (d, 2H, J = 4 Hz, Ar-H), 8.27 (d, 2H, J = 8 Hz, Ar-H). 13C-NMR (DMSO-d6, δ ppm): 56.45, 69.74, 101.84, 108.59, 112.26, 114.14, 124.04, 127.66, 143.13, 146.72, 147.82, 149.55, 151.01, 161.13. MS (m/z): [M]+ 327. Anal. Calcd. for C17H13NO6: C, 62.39%; H: 4.00%; N: 4.28%. Found: C, 62.02%; H, 4.15%; N, 4.49%.
7-(3,4-Dichlorobenzyloxy)-6-methoxy-2H-chromen-2-one (22)
White granular crystals; 53.08% yield; m.p. 151.5–152.3 °C; IR νmax (KBr) cm−1: 2948, 1698, 1607, 1564, 1509, 1435, 1375, 1274, 1259, 1139, 1018, 860. 1H-NMR (DMSO-d6, δ ppm): 3.95 (s, 3H, OCH3), 5.14 (s, 2H, CH2O), 6.27 (d, 1H, J = 8 Hz, C3-H), 6.85 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.47 (d, 1H, J = 8 Hz, C4-H), 7.56–7.64 (m, 3H, Ar-H). 13C-NMR (DMSO-d6, δ ppm): 56.42, 69.67, 101.79, 108.48, 112.08, 113.94, 126.54, 129.22, 130.78, 133.02, 135.87, 143.36, 144.04, 146.71, 149.72, 151.21, 161.27. MS (m/z): [M]+ 351. Anal. Calcd. for C17H12Cl2O4: C, 58.14%; H: 3.44%. Found: C, 58.95%; H, 3.91%.
7-(4-Chlorobenzyloxy)-6-methoxy-2H-chromen-2-one (23)
White powder; 76.13% yield; m.p. 171.8–172.3 °C; IR νmax (KBr) cm−1: 3074, 3014, 2949, 2927, 2875, 1705, 1615, 1562, 1515, 1461, 1426, 1393, 1279, 1249, 1145, 1015, 880. 1H-NMR (DMSO-d6, δ ppm): 3.81 (s, 3H, OCH3), 5.21 (s, 2H, CH2O), 6.32 (d, 1H, J = 8 Hz, C3-H), 7.18 (s, 1H, C8-H), 7.30 (s, 1H, C5-H), 7.48, 7.51 (dd, 4H, J = 8 Hz, Ar-H), 7.97 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 56.38, 69.80, 101.72, 109.60, 111.98, 113.38, 129.04, 130.34, 133.23, 135.69, 144.77, 146.48, 149.60, 151.57, 160.99. MS (m/z): [M]+ 316. Anal. Calcd. for C17H13ClO4: C: 64.47%; H: 4.14%. Found: C, 64.60%; H, 4.18%.
7-(4-(Trifluoromethyl)benzyloxy)-6-methoxy-2H-chromen-2-one (24)
White needle-like crystals; 83.56% yield; m.p. 192.8–193.3 °C; IR νmax (KBr) cm−1: 3073, 2938, 1713, 1616, 1565, 1515, 1463, 1427, 1393, 1281, 1250, 1146, 1019, 881. 1H-NMR (DMSO-d6, δ ppm): 3.94 (s, 3H, OCH3), 5.26 (s, 2H, CH2O), 6.29 (d, 1H, J = 8 Hz, C3-H), 6.90 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.56-7.67 (m, 5H, Ar-H and C4-H). 13C-NMR (DMSO-d6, δ ppm): 56.47, 70.22, 101.82, 108.53, 112.03, 113.95, 125.77, 125.81, 127.35, 130.39, 139.71, 143.19, 146.75, 149.65, 151.36, 161.23. MS (m/z): [M]+ 350. Anal. Calcd. for C18H13F3O4: C, 61.72%; H, 3.74%. Found: C, 62.35%; H, 4.27%.
7-(4-(Trifluoromethoxy)benzyloxy)-6-methoxy-2H-chromen-2-one (25)
White powder; 84.32% yield; m.p. 127.0–127.2 °C; IR νmax (KBr) cm−1: 3056, 2960, 1727, 1615, 1565, 1514, 1466, 1426, 1382, 1248, 1147, 1023, 872. 1H-NMR (DMSO-d6, δ ppm): 3.92 (s, 3H, OCH3), 5.18 (s, 2H, CH2O), 6.27 (d, 1H, J = 8 Hz, C3-H), 6.89 (s, 1H, C8-H), 7.24 (d, 2H, J = 8 Hz, Ar-H), 7.28 (s, 1H, C5-H), 7.49 (d, 2H, J = 8 Hz, Ar-H), 7.62 (d, 1H, J = 12Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 56.40, 70.17, 101.68, 108.48, 111.92, 113.74, 119.16, 121.26, 128.86, 134.40, 143.28, 146.73, 149.11, 149.65, 151.50, 161.28. MS (m/z): [M]+ 366. Anal. Calcd. for C18H13F3O5: C, 59.02%; H, 3.58%. Found: C, 59.87%; H, 4.26%.
3.2.3. General Procedure for the Synthesis of 14–17
K2CO3 (0.2073 g, 3 mmol) were added into a solution of scopoletin (4, 0.3843 g, 2 mmol) in DMF (10 mL), and the mixture was reacted under microwave irradiation (80 W power) for 7 min. Then the naphthenic halide (3 mmol) was added into the mixture and reacted under microwave irradiation (120 W power) for 40 min (the reaction progress was monitored by TLC with UV detection). After cooling the reaction and poured into 15 mL water, the mixture liquor was extracted with ethyl acetate. The ethyl acetate layers were pooled and washed with saturation salt solution, dried over anhydrous sodium sulfate, evaporated under reduced pressure to give the target crude products. The crude products were purified by column chromatography using petroleum ether/ethyl acetate from 10:1 to 7:1 as the gradient eluent system to yield the products 14–17.
7-(Cyclopropylmethoxy)-6-methoxy-2H-chromen-2-one (14)
Brown sheet-like crystals; 74.59% yield; m.p. 149.2–149.6 °C; IR νmax (KBr) cm−1: 3076, 2963, 2937, 2880, 1715, 1615, 1563, 1462, 1426, 1387, 1276, 1248, 1146, 1024, 880. 1H-NMR (DMSO-d6, δ ppm): 0.39 –0.43 (m, 2H, CH2), 0.68–0.73 (m, 2H CH2), 1.32–1.42 (m, 1H, CH), 3.91(d, 2H, J = 4 Hz, CH2), 3.92 (s, 3H, OCH3), 6.28 (d, 1H, J = 12 Hz, C3-H), 6.86 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.62 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 3.58, 9.89, 56.42, 74.19, 100.94, 108.23, 111.33, 113.32, 143.39, 146.58, 149.93, 152.35, 161.49; MS (m/z): [M]+ 246. Calcd. for C14H14O4: C, 68.26%; H, 5.69%. Found: C, 68.38%; H, 5.81%.
7-(Cyclopentyloxy)-6-methoxy-2H-chromen-2-one (15)
White needle-like crystals; 72.89% yield; m.p. 80.7–81.2 °C; IR νmax (KBr) cm−1: 3075, 2944, 2867, 1718, 1611, 1558, 1511, 1467, 1425, 1387, 1277, 1248, 1144, 1024, 875. 1H-NMR (DMSO-d6, δ ppm): 1.64–1.70 (m, 2H, CH2), 1.82–1.87 (m, 2H, CH2), 1.89–1.94 (m, 2H, CH2), 1.98–2.07 (m, 2H, CH2), 3.88 (s, 3H, OCH3), 4.79-4.84(m, 1H, CH), 6.27 (d, 1H, J = 12 Hz, C3-H), 6.84 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.62 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 24.23, 32.78, 56.49, 81.03, 102.06, 108.51, 111.07, 113.07, 143.45, 146.99, 149.95, 151.76, 161.60. MS (m/z): [M]+ 260. Calcd. for C15H16O4: C, 69.23%; H, 6.15%. Found: C, 69.32%; H, 6.24%.
7-(Cyclohexyloxy)-6-methoxy-2H-chromen-2-one (16)
White granular crystals; 12.21% yield; m.p. 141.9–142.0 °C; IR νmax (KBr) cm−1: 3075, 2944, 2840, 1716, 1608, 1563, 1514, 1456, 1425, 1387, 1282, 1248, 1144, 1024, 873. 1H-NMR (DMSO-d6, δ ppm): 1.26–1.45 (m, 2H, CH2), 1.57–1.66 (m, 2H, CH2), 1.83–1.88 (m, 2H, CH2), 2.06–2.09 (m, 1H, CH), 3.89 (s, 3H, OCH3), 6.27 (d, 1H, J = 8 Hz, C3-H), 6.86 (s, 1H, C8-H), 7.27 (s, 1H, C5-H),7.61 (d, 1H, J = 12 Hz, C4-H) (Figure S7). 13C-NMR (DMSO-d6, δ ppm): 23.91, 25.44, 31.50, 56.56, 77.03, 102.35, 108.81, 111.27, 113.21, 143.30, 147.26, 150.00, 151.32, 161.49 (Figure S8). MS (m/z): [M + H]+ 275. Calcd. for C16H18O4: C, 70.07%; H, 6.57%. Found: C, 70.15%; H, 6.67%.
7-(Cyclohexylmethoxy)-6-methoxy-2H-chromen-2-one (17)
White granular crystals; 30.23% yield; m.p. 144.4–144.7 °C; IR νmax (KBr) cm−1: 3072, 2938, 2854, 1713, 1614, 1562, 1514, 1464, 1425, 1385, 1279, 1251, 1144, 1034, 882. 1H-NMR (DMSO-d6, δ ppm): 1.02–1.11 (m, 2H, CH2), 1.20-1.32 (m, 4H, 2 × CH2), 1.70–1.79 (m, 4H, 2 × CH2), 1.89-1.97 (m, 1H, CH), 3.86 (d, 2H, J = 4 Hz, CH2), 3.90 (s, 3H, OCH3), 6.27 (d, 1H, J = 8 Hz, C3-H), 6.85 (s, 1H, C8-H), 7.28 (s, 1H, C5-H), 7.62 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 25.66, 26.40, 29.81, 37.16, 56.54, 74.65, 100.80, 108.48, 111.17, 113.16, 143.41, 146.66, 150.04, 152.72, 161.53. MS (m/z): [M]+ 288. Calcd. for C17H20O4: C, 70.83%; H, 6.94%. Found: C, 70.73%; H, 6.99%.
3.2.4. General Procedure for the Synthesis of 26–37
Triethylamine (0.3643 g, 3.6 mmol) was added to a solution of the appropriate alkylamine or substituted benzylamine 5a–l (3 mmol) in dichloromethane (7.5 mL), and the reaction mixture was stirred for 5 min at room temperature, then 2-chloroacetyl chloride (0.3857 g, 3.6 mmol) was added dropwise to this reaction mixture at 0 °C and stirred for 15 min at room temperature. After completion of the reaction, the solvent was evaporated under reduced pressure to afford 6a–l. KI (0.5976 g, 3.6 mmol) and CTAB (98.40 mg, 7.5% mmol) were added to a solution of the crude product 6a–l in acetone (30 mL) and maintained stirring at reflux for 2 h to afford 7a−l. K2CO3 (0.2073 g, 3 mmol) was added to a solution of scopoletin (0.3843 g, 2 mmol) in acetone (30 mL), and the reaction mixture was stirred at refluxed for 30 min. Then crude intermediates 7a–l were added into the mixture and maintained reflux for 8–12 h (the reaction progress was monitored by TLC with UV detection). After cooling the reaction and filtration, the solvent was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate, washed with saturation sodium bicarbonate, and saturation salt solution successively, dried over anhydrous sodium sulfate, evaporated under reduced pressure to give the target crude products. The crude products were purified by column chromatography using petroleum ether/ethyl acetate from 6:1 to 2:1 as the gradient eluent system to yield the products 26–37.
2-(6-Methoxy-2-oxo-2H-chromen-7-yloxy)-N-methylacetamide (26)
White needle-like crystals; 21.22% yield; m.p. 155.8–156.40 °C; IR νmax (KBr) cm−1: 3327, 2982, 1750, 1704, 1609, 1566, 1515, 1425, 1394, 1280, 1251, 1144, 1019, 854. 1H-NMR (CDCl3, δ ppm): 3.83 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 4.78 (s, 2H, CH2O), 6.32 (d, 1H, J = 12 Hz, C3-H), 6.91 (s, 1H, C8-H), 7.29 (s, 1H, C5-H), 7.64 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 26.48, 52.61, 65.82, 101.45, 108.72, 112.56, 114.21, 143.23, 146.53, 149.40, 150.66, 161.21, 168.21. MS (m/z): [M + H]+ 264. Anal. Calcd. for C13H13NO5: C, 59.31%; H, 4.98%; N, 5.32%. Found: C, 60.40%; H, 4.60%; N, 5.20%.
N-Ethyl-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (27)
White granular crystals; 30.28% yield; m.p. 171.2–172.3 °C; IR νmax (KBr) cm−1: 3409, 2933, 1713, 1683, 1591, 1512, 1442, 1421, 1354, 1266, 1243, 1148, 1002, 881. 1H-NMR (DMSO-d6, δ ppm): 1.19 (t, 3H, J = 8 Hz, CH3), 3.37–3.41 (m, 2H, CH2), 3.92 (s, 3H, OCH3), 4.53 (s, 2H, CH2O), 6.30 (d, 1H, J = 12 Hz, C3-H), 6.90 (s, 1H, C8-H), 7.29 (s, 1H, C5-H), 7.63 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 14.78, 34.05, 56.29, 68.59, 102.59, 108.58, 112.86, 114.50, 143.08, 146.56, 149.39, 150.22, 160.99, 166.94. MS (m/z): [M]+ 277. Anal. Calcd. for C14H15NO5: C, 60.64%; H, 5.45%; N, 5.05%. Found: C, 60.86%; H, 5.45%; N, 4.65%.
2-(6-Methoxy-2-oxo-2H-chromen-7-yloxy)-N-propylacetamide (28)
White granular crystals; 34.42% yield; m.p. 173.2–173.8 °C; IR νmax (KBr) cm−1: 3422, 3053, 2962, 2876, 1717, 1673, 1611, 1563, 1510, 1427, 1390, 1278, 1251, 1143, 1023, 889. 1H-NMR (DMSO-d6, δ ppm): 0.96 (t, 3H, J = 8 Hz, CH3), 1.55–1.64 (m, 2H, CH2), 3.31–3.36 (m, 2H, CH2), 3.95 (s, 3H, OCH3), 4.57 (s, 2H, CH2O), 6.33 (d, 1H, J = 12 Hz, C3-H), 6.94 (s, 1H, C8-H), 7.31 (s, 1H, C5-H), 7.66 (d, 1H, J = 12Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 11.30, 22.75, 40.77, 56.30, 68.63, 102.62, 108.60, 112.87, 114.51, 143.10, 146.58, 149.40, 150.25, 160.99, 167.03. MS (m/z): [M]+ 291. Anal. Calcd. for C15H17NO5: C, 61.85%; H, 5.88%; N, 4.81%. Found: C, 62.05%; H, 5.86%; N, 4.70%.
N-Isopropyl-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (29)
White granular crystals; 19.52% yield; m.p. 197.2–197.5 °C; IR νmax (KBr) cm−1: 3305, 3077, 2967, 2875, 1722, 1663, 1613, 1558, 1507, 1463, 1422, 1396, 1275, 1248, 1151, 1034, 872. 1H-NMR (DMSO-d6, δ ppm): 1.23 (d, 6H, J = 8 Hz, CH3), 3.95 (s, 3H, OCH3), 4.13–4.25 (m, 1H, CH), 4.55 (s, 2H, CH2O), 6.35 (d, 1H, J = 8 Hz, C3-H), 6.93 (s, 1H, C8-H), 7.28 (s, 1H, C5-H), 7.65 (d, 1H, J = 12Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 22.68, 41.24, 56.32, 69.00, 103.16, 108.68, 113.02, 114.74, 142.94, 146.69, 149.50, 150.40, 160.94, 166.25. MS (m/z): [M]+ 291. Anal. Calcd. for C15H17NO5: C, 61.85%; H, 5.88%; N, 4.81%. Found: C, 60.90%; H, 5.82%; N, 4.65%.
N-Butyl-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (30)
White powder; 19.59% yield; m.p. 168.4–168.5 °C; IR νmax (KBr) cm−1: 3426, 3055, 2959, 2934, 2862, 1721, 1675, 1614, 1565, 1511, 1464, 1427, 1391, 1280, 1252, 1146, 1027, 891. 1H-NMR (DMSO-d6, δ ppm): 0.93-0.96 (t, 3H, CH3), 1.31–1.42 (m, 2H, CH2), 1.51–1.60 (m, 2H, CH2), 3.34-3.39 (m, 2H, CH2), 3.93 (s, 3H, OCH3), 4.56 (s, 2H, CH2O), 6.34 (d, 1H, J = 8 Hz, C3-H), 6.91 (s, 1H, C8-H), 7.27 (s, 1H, C5-H), 7.63 (d, 1H, J = 8Hz, C4-H) (Figure S11). 13C-NMR (DMSO-d6, δ ppm): 13.74, 20.01, 31.51, 38.86, 56.32, 69.70, 102.78, 108.63, 112.92, 114.64, 143.00, 146.61, 149.48, 150.28, 160.98, 167.02 (Figure S12). MS (m/z): [M]+ 305. Anal. Calcd. for C16H19NO5: C, 62.95%; H, 6.23%; N, 4.59%. Found: C, 63.15%; H, 6.30%; N, 4.60%.
N-Benzyl-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (31)
White powder; 38.02% yield; m.p. 84.50–86.20 °C; IR νmax (KBr) cm−1: 3420, 3053, 2931, 2872, 1717, 1671, 1612, 1565, 1508, 1460, 1423, 1389, 1276, 1249, 1143, 1018, 885. 1H-NMR (DMSO-d6, δ ppm): 3.93 (s, 3H, OCH3), 4.09 (d, 2H, J = 8 Hz, CH2), 4.53 (s, 2H, CH2O), 6.29 (d, 1H, J = 12 Hz, C3-H), 6.91 (s, 1H, C8-H), 7.28 (s, 1H, C5-H), 7.49–7.66 (m, 5H, Ar-H), 8.20 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 44.58, 56.43, 66.63, 101.66, 108.39, 111.92, 113.73, 124.06, 127.70, 128.91, 131.00, 143.43, 146.72, 149.63, 151.50, 161.46, 167.79. MS (m/z): [M]+ 339. Anal. Calcd. for C19H17NO5: C, 67.25%; H, 5.05%; N, 4.13%. Found: C, 67.28%; H, 4.40%; N, 4.91%.
N-(3-Chlorobenzyl)-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)actamide (32)
White powder; 11.61% yield; m.p. 155.6–156.0 °C; IR νmax (KBr) cm−1: 3420, 3053, 2931, 2850, 1716, 1671, 1612, 1567, 1508, 1464, 1423, 1389, 1276, 1248, 1145, 1020, 881. 1H-NMR (DMSO-d6, δ ppm): 3.88 (s, 3H, OCH3), 4.44 (d, 2H, J = 4 Hz, CH2), 4.53 (s, 2H, CH2O), 6.28 (d, 1H, J = 8 Hz, C3-H), 6.87 (s, 1H, C8-H), 7.03 (s, 1H, C5-H), 7.29–7.40 (m, 3H, Ar-H), 7.46(s, 1H, Ar-H), 7.92 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 41.79, 56.77, 68.84, 103.20, 107.47, 111.51, 113.42, 126.32, 127.08, 127.68, 130.01, 133.66, 141.07, 143.36, 144.02, 149.70, 150.25, 161.51, 167.30. MS (m/z): [M]+ 373. Anal. Calcd. for C19H16ClNO5: C, 61.13%; H, 4.29%; N, 3.75%. Found: C, 61.87%; H, 4.85%; N, 3.43%.
N-(4-Chlorobenzyl)-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (33)
White sheet-like crystals; 30.12% yield; m.p. 178.3–179.2 °C; IR νmax (KBr) cm−1: 3420, 3054, 2932, 1716, 1671, 1614, 1566, 1507, 1461, 1423, 1388, 1276, 1248, 1144, 1017, 884. 1H-NMR (DMSO-d6, δ ppm): 3.83 (s, 3H, OCH3), 4.53 (d, 2H, J = 4Hz, CH2), 4.63 (s, 2H, CH2O), 6.35 (d, 1H, J = 12 Hz, C3-H), 6.88 (s, 1H, C8-H), 7.16 (s, 1H, C5-H), 7.23–7.32 (m, 4H, Ar-H), 7.63 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 42.42, 56.18, 68.92, 103.16, 108.60, 113.12, 114.84, 128.91, 129.12, 133.60, 136.21, 142.94, 146.60, 149.40, 150.11, 160.91, 167.28. MS (m/z): [M]+ 373. Anal. Calcd. for C19H16ClNO5: C, 61.05%; H, 4.31%; N, 3.75%. Found: C, 61.28%; H, 4.40%; N, 3.59%.
N-(3,4-Dichlorobenzyl)-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (34)
White powder; 36.12% yield; m.p. 161.15–162.25 °C; IR νmax (KBr) cm−1: 3417, 3040, 2996, 1724, 1678, 1612, 1564, 1507, 1472, 1425, 1389, 1274, 1252, 1145, 1024, 878. 1H-NMR (DMSO-d6, δ ppm): 3.87 (s, 3H, OCH3), 4.36 (d, 2H, J = 8 Hz, CH2), 4.51 (s, 2H, CH2O), 6.35 (d, 1H, J = 12 Hz, C3-H), 6.93 (s, 1H, C8-H), 7.30 (s, 1H, C5-H), 7.36–7.41 (m, 3H, Ar-H), 7.68 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 42.18, 56.32, 68.84, 103.04, 108.76, 113.16, 114.68, 127.61, 129.86, 130.63, 131.41, 132.41, 137.78, 143.19, 146.58, 149.33, 150.06, 162.90, 167.47. MS (m/z): [M − H]+ 407. Anal. Calcd. for C19H15Cl2NO5: C, 55.90%; H, 3.70%; N, 3.43%. Found: C, 55.50%; H, 3.44%; N, 3.55%.
N-(4-Methylbenzyl)-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (35)
White sheet-like crystals; 65.36% yield; m.p. 171.4–171.6 °C; IR νmax (KBr) cm−1: 3430, 3055, 2931, 1727, 1677, 1614, 1567, 1507, 1464, 1427, 1389, 1276, 1250, 1146, 1024, 881. 1H-NMR (DMSO-d6, δ ppm): 2.34 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 5.52 (d, 2H, J = 4 Hz, CH2), 4.62 (s, 2H, CH2O), 6.33 (d, 1H, J = 8 Hz, C3-H), 6.87 (s, 1H, C8-H), 7.15, 7.20 (dd, 4H, J = 8 Hz, Ar-H), 7.27 (s, 1H, C5-H), 7.63 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 21.13, 42.88, 56.15, 69.01, 103.18, 108.61, 113.06, 114.73, 127.74, 129.43, 134.62, 137.41, 142.96, 146.67, 149.42, 150.29, 160.92, 167.11. MS (m/z): [M]+ 353. Anal. Calcd. for C20H19NO5: C, 67.98%; H, 5.42%; N, 3.96%. Found: C, 67.69%; H, 5.46%; N, 3.85%.
N-(4-Methoxybenzyl)-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (36)
White sheet crystal; 80.34% yield; m.p. 161.3–161.6 °C; IR νmax (KBr) cm−1: 3430, 3054, 1727, 1675, 1611, 1567, 1507, 1426, 1388, 1276, 1249, 1144, 1024, 880. 1H-NMR (DMSO-d6, δ ppm): 3.80 (s, 6H, OCH3), 4.48 (d, 2H, J = 4 Hz, CH2), 4.61 (s, 2H, CH2O), 6.32 (d, 1H, J = 12 Hz, C3-H), 6.84–6.88 (m, 2H, Ar-H), 7.11 (s, 1H, C8-H), 7.22 (d, 2H, J = 8 Hz, Ar-H), 7.28 (s, 1H, C5-H), 7.61 (d, 1H, J = 8 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 42.60, 55.33, 56.16, 69.03, 103.18, 108.63, 113.05, 114.10, 114.68, 129.12, 129.74, 142.95, 146.66, 149.39, 150.29, 159.12, 160.89, 167.07. MS (m/z): [M]+ 369. Anal. Calcd. for C20H19NO6: C, 65.03%; H, 5.19%; N, 3.79%. Found: C, 65.20%; H, 5.24%; N, 3.66%.
N-(4-tert-Butylbenzyl)-2-(6-methoxy-2-oxo-2H-chromen-7-yloxy)acetamide (37)
White powder; 50.34% yield; m.p.177.5–178.1 °C; IR νmax (KBr) cm−1: 3430, 2961, 1730, 1682, 1615, 1568, 1520, 1444, 1428, 1390, 1279, 1249, 1147, 1025, 880. 1H-NMR (DMSO-d6, δ ppm): 1.28 (s, 9H, 3 × CH3), 3.79 (s, 3H, OCH3), 4.39 (d, 2H, J = 8 Hz, CH2), 4.61 (s, 2H, CH2O), 6.32 (d, 1H, J = 8 Hz, C3-H), 6.90 (s, 1H, C8-H), 7.24 (d, 2H, J = 8 Hz, Ar-H), 7.31 (s, 1H, C5-H), 7.37 (d, 2H, J = 8 Hz, Ar-H), 7.66 (d, 1H, J = 12 Hz, C4-H). 13C-NMR (DMSO-d6, δ ppm): 31.34, 42.75, 42.88, 56.17, 69.04, 103.15, 108.71, 113.09, 114.62, 125.51, 127.66, 134.40, 143.13, 146.68, 149.38, 150.71, 150.29, 160.98, 167.15. MS (m/z): [M]+ 395. Anal. Calcd. for C23H25NO5: C, 69.86%; H, 6.37%; N, 3.54%. Found: C, 69.91%; H, 6.91%; N, 3.59%.
3.3. Acaricidal Activity Assay
T. cinnabarlnus was reared on potted young cowpea plants in the laboratory at (26 ± 1) °C and (70 ± 10) % relative humidity (R. H.) and a 14 h:10 h (light:dark) cycle with no acaricide exposure for at least 15 years, which originally collected from field young cowpea plants in Beibei District, Chongqing Municipality, China.
The slide-dip method [52] was adopted to evaluate the acaricidal activity of 8–37 against female adults of T. cinnabarinus. The appropriate amounts of target compounds were dissolved in 0.2 mL acetone and then diluted with water containing 0.1% Tween-80 to obtain the desired final concentration of 1000 mg/L for the preliminary screening. Based on the preliminary test results, a series of five to seven concentrations of the tested compounds were chosen to determine the median lethal concentration (LC50) values of the compounds. Propargite 90.00% TC and scopoletin were used as positive controls, and water containing 0.1% Tween-80 was used as a blank control. Acaricidal activity assays were performed in triplicate and repeated thrice. The LC50 values of the tested compounds were calculated using the probit analysis procedure of SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
The leaf-dip method was used to evaluate the acaricidal activity of compound 8 against eggs, larval, and nymphal of T. cinnabarinus. The test solutions of compound 8 was prepared as above slide-dip method. Leaf discs were prepared to obtain uniform individuals at different developmental stages. Fresh cowpea leaves that had not been exposed to pesticides were washed thoroughly. Leaf discs with 3 cm diameters were placed on a corresponding size water-saturated sponge in a Petri dish (9 cm diameter) [53]. Adult females (20–30) were transferred to each leaf disc, allowed to lay eggs, and removed after 12 h. The leaf disc with eggs, larvae, and nymphs were then dipped in the compound 8 solutions for 5 s, taken out, and then laid on sponge in Petri dish again. The observed results were recorded after 48 h.
3.4. 2D- and 3D-QSAR Study
3.4.1. Data Set
The synthesized thirty target compounds and their acaricidal activities (LC50 values) were used as data set for QSAR analysis. They were randomly divided into a 25-molecule training set for 2D- and 3D-QSAR models development and 5-molecule test set (compounds 11, 16, 19, 27 and 33) for external validation.
3.4.2. 2D-QSAR (Multiple Linear Regression Model) Method
2D structures of the 30 target synthesized compounds were generated by ChemDraw Ultra (Cambridge Soft Corporation, Cambridge, MA, USA), and their energies were minimized using MM2 of Chem3D Ultra. Then 1666 molecular descriptors were calculated for each compound using DRAGON Web version 1.0 developed by the Milano Chemometrics and QSAR Research Group (http://www.vcclab.org/lab/edragon/start.html). These descriptors included (i) 0D constitutional (atom and group counts), (ii) 1D functional groups and atom-centred fragments, (iii) 2D topological, counts, autocorrelations, connectivity indices, information indices, topological indices, and eigenvalue-based indices, and (iv) 3D geometrical, WHIM, and GETAWAY descriptors, etc. [54]. 1302 descriptors were utilized as input values for model construction after eliminating the descriptors with constant values or mostly zero values (>90%) from the all the calculated descriptors.
2D-QSAR models were obtained using SPSS software (Version 17.0) that can run multiple linear regression. Different mathematical transformations of the observed median lethal concentration (LC50) of the training set analogs, including property LC50 (mg/L), LC50 (mol/L), 1/LC50 (mg/L), 1/LC50 (mol/L), log LC50 (mg/L), log LC50 (mol/L), −log LC50 (mg/L) and −log LC50 (mol/L) values, were utilized in the present 2D-QSAR modeling to searching for the best model. −log LC50 (mol/L) (pLC50) values were used as dependent variables. Stepwise method for variable selection along with multiple linear regression was used to construct models.
3.4.3. 3D-QSAR (CoMFA and CoMSIA) Methods
The molecular structures of synthesized compounds were generated and optimized using SYBYL 6.9 (Tripos Associates, St. Louis, MO, USA). The Gasteiger–Hückel charge, Tripos force field, and Powell method were used for structure optimization. To guarantee the obtaining of the molecular lowest energy conformation, conformation search was executed by using multisearch routin [55]. The most important component of a 3D-QSAR study is the alignment of the molecules based on the scaffold they share [56]. In this paper, the 7-oxy-6-methoxy-2H-chromen-2-one structure was selected as the common scaffold for molecular alignment. Compound 8 was used as the template molecule. All other synthesized acaricidal agents were aligned with the 7-oxy-6-methoxy-2H-chromen-2-one core.
The comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) are commonly used 3D-QSAR methods [51]. In CoMFA, the steric and electrostatic fields were calculated by setting the energy cutoff as the default value of 30 kcal·mol−1. Five CoMSIA fields including the steric, electrostatic, hydrophobic, hydrogen-bond donor and hydrogen-bond acceptor were calculated using the default attenuation factor of 0.3 for Gaussian function. Field type “Stdev * Coeff” was used as the coefficient to analysis the contour map of each field [36]. The partial least squares (PLS) [57] was used to quantify the relationships by setting the biological activity (pLC50 values) as the dependent variables and the CoMFA/CoMSIA descriptors as independent variables.
3.5. Molecular Docking
Molecular docking studies were performed using AutoDock 4.2 and AutoDock Tools version 1.5.6 (ADT). The 3D structure of TcPMCA1 (GenBank No. KP455490), and its binding pocket were obtained from the I-TASSER server (Available online: http://zhanglab.ccmb.med.umich.edu/I-TASSER/), then water molecules were removed, polar hydrogen atoms were added, Compute Gasteiger charges were added, and AD 4 type atoms were assigned [41]. The 3D structure of ligands were constructed and their energy minimization were performed using ChemOffice 2004. Following by the structural optimization, all ligands were prepared for docking by merging non-polar hydrogen atoms, detecting rotatable bonds and adding gasteiger charges [41]. The grid box size of 60 × 60 ×60 Å was generated and allocated to center of binding cavity using x, y and z coordinates of 102.273, 100.115, and 118.080 for intend searching modality. Other parameters were set as the default. The Lamarckian genetic algorithmwas applied to calculate the possible conformation of the ligand molecule and macromolecule. Finally, the docking results were analyzed using the free version of Discovery Studio Visualizer 4.5 (Accelrys Software Inc., San Diego, CA, USA) [58].
3.6. In Silico ADME Prediction
On the basis of Lipinski’s rule of five and its extensions [59], we calculated molecular volume (MV), molecular weight (MW), logarithm of partition coefficient (miLogP), number of hydrogen bond acceptors (n-ON), number of hydrogen bonds donors (n-OHNH), topological polar surface area (TPSA), number of rotatable bonds (n-ROTB) and Lipinski’s rule of five using Molinspiration online property calculation toolkit [60]. Absorption (% ABS) was calculated as follows: % ABS = 109 − (0.345 × TPSA) [61].
4. Conclusions
Thirty phenolic ether derivatives of scopoletin including twelve compounds with amide groups were synthesized successfully using a molecular hybridization method. Their acaricidal activities, QSAR, molecular docking and a silico ADME properties were investigated. Some of these compounds exhibit more pronounced acaricidal activity than scopoletin, especially compounds 32, 20, 28, 27 and 8 exhibited about 8.41-, 7.32-, 7.23-, 6.76-, and 6.65-fold higher acaricidal potency than scopoletin. Compound 32 possessed the the most promising acaricidal activity and exhibited about 1.45-fold higher acaricidal potency against T. cinnabarinus than propargite. Statistically significant 2D-QSAR model supports the observed acaricidal activities and reveals that polarizability (HATS5p) was the most important parameter controlling bioactivity. 3D-QSAR (CoMFA: q2 = 0.802, r2 = 0.993; CoMSIA: q2 = 0.735, r2 = 0.965) results show that bulky substituents at R4, R1, R2 and R5 (C6, C3, C4, and C7) positions, electron positive groups at the R5 (C7) position, hydrophobic groups at the R1 (C3) and R2 (C4), H-bond donors groups at R1 (C3) and R4 (C6) will increase their acaricidal activity, which provide a good insight into the molecular features relevant to the acaricidal activity for further designing novel acaricidal agents. Molecular docking demonstrates that these selected derivatives display different bide modes with TcPMCA1 from lead compound and they interact with more key amino acid residues than scopoletin. In silico ADME properties study of scopoletin and its phenolic ether derivatives were also analyzed and showed potential to develop these compounds as good acaricidal candidates.
Supplementary Materials
1H-NMR and 13C-NMR of representive compounds.
Author Contributions
Jinxiang Luo, Ting Lai, Wei Ding and Yongqiang Zhang conceived and designed the experiments; Jinxiang Luo, Ting Lai, Tao Guo, Fei Chen performed the experiments and analyzed the data; Jinxiang Luo wrote the paper; Jinxiang Luo, Linli Zhang, Wei Ding and Yongqiang Zhang revised the paper.
Acknowledgments
We are grateful to Yuwei Wang in School of Pharmacy, Lanzhou University for Molecular Docking and 3D-QSAR analysis. This work was supported by the Chinese National Nature Science Foundation (31272058, 31572041 and 31601674), Chongqing Municipal Natural Science Foundation of China (cstc2016jcyjA0501), and the Fundamental Research Fund for the Central Universities of China (No. XDJK2014C183).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Luo, J.X.; Ding, W.; Zhang, Y.Q.; Yang, Z.G.; Li, Y.; Ding, L.J. Semisynthesis and acaricidal activities of isoxazole and pyrazole derivatives of a natural product bisdemethoxycurcumin. J. Pestic. Sci. 2013, 38, 214–219. [Google Scholar] [CrossRef]
- Zhang, J.P.; Wang, J.J.; Zhao, Z.M.; Dou, W.; Chen, Y. Effects of simulated acid rain on the physiology of carmine spider mite, Tetranychus cinnabarinus (Boisduvals) (Acari: Tetranychidae). J. Appl. Entomol. 2004, 128, 342–347. [Google Scholar] [CrossRef]
- Çakmak, İ.; Başpinar, H.; Madanlar, N. Control of the carmine spider mite Tetranychus cinnabarinus Boisduval by the predatory mite Phytoseiulus persimilis (Athias-Henriot) in protected strawberries in Aydin, Turkey. Turk. J. Agri. For. 2005, 29, 259–265. [Google Scholar]
- Sertkaya, E.; Kaya, K.; Soylu, S. Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite (Tetranychus cinnabarinus Boisd.) (Acarina: Tetranychidae). Ind. Crop. Prod. 2010, 31, 107–112. [Google Scholar] [CrossRef]
- Sances, F.V.; Wyman, J.A.; Ting, I.P.; Van Steenwyk, R.A.; Oatman, E.R. Spider mite interactions with photosynthesis, transpiration and productivity of strawberry. Environ. Entomol. 1981, 10, 442–448. [Google Scholar] [CrossRef]
- Bi, J.L.; Niu, Z.M.; Yu, L.; Toscano, N.C. Resistance status of the carmine spider mite, Tetranychus cinnabarinus and the twospotted spider mite, Tetranychus urticae to selected acaricides on strawberries. Insect Sci. 2016, 23, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.X.; Ding, W.; Zhang, Y.Q.; Yang, Z.G.; Li, Y. Synthesis and acaricidal activity of curcumin isoxazole and pyrazole derivatives. Chin. J. Pestic. Sci. 2013, 15, 372–380. [Google Scholar]
- He, L.; Xue, C.H.; Wang, J.J.; Li, M.; Lu, W.C.; Zhao, Z.M. Resistance selection and biochemical mechanism of resistance to two acaricides in Tetranychus cinnabarinus (Boiduval). Pestic. Biochem. Phys. 2009, 93, 47–52. [Google Scholar]
- Yu, H.H.; Yue, Y.; Dong, X.L.; Li, R.F.; Li, P.C. The acaricidal activity of venom from the Jellyfish Nemopilema nomurai against the carmine spider mite Tetranychus cinnabarinus. Toxins 2016, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.D.; Tuttle, D.M. A Guide to Spider Mites (Acari: Tetranychidae) from the United States; Indira Publishing House: West Bloomfield, MI, USA, 1994. [Google Scholar]
- Wu, K.M.; Liu, X.C.; Qin, X.Q.; Lou, G.Q. Investigation of carmine spider mite (Tetranychus cinnabarinus) resistance to insecticides. Acta Agric. Boreali-Sin. 1990, 5, 117–123. [Google Scholar]
- Ambikadevi, D.; Samarjit, R. Chemical control of red spider mite Tetranychus cinnabarinus (Boisduval) on okra. J. Trop. Agric. 1997, 35, 38–40. [Google Scholar]
- Herron, G.A.; Edge, V.E.; Wilson, L.J.; Rophail, J. Organophosphate resistance in spider mites (Acari: Tetranychidae) from cotton in Australia. Exp. Appl. Acarol. 1998, 22, 17–30. [Google Scholar] [CrossRef]
- Guo, F.Y.; Zhang, Z.Q.; Zhao, Z.M. Pesticide resistance of Tetranychus cinnabarinus (Acari: Tetranychidae) in China: A review. Syst. Appl. Acarol. 1998, 3, 3–7. [Google Scholar] [CrossRef]
- Wei, J.; Ding, W.; Zhao, Y.G.; Vanichpakorn, P. Leaf extracts against Tetranychus cinnabarinus (Boisduval) (Acarina: Tetranychidae). J. Asia-Pac. Entomol. 2011, 14, 353–356. [Google Scholar] [CrossRef]
- Pan, R.; Gao, X.H.; Lu, D.; Xu, X.X.; Xia, Y.F.; Dai, Y. Prevention of FGF-2-induced angiogenesis by scopoletin, a coumarin compound isolated from Erycibe obtusifolia Benth, and its mechanism of action. Int. Immunopharmacol. 2011, 11, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Liu, F.; Lu, Y.H.; Cheng, C.H.K.; Wang, Z.T. Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2003, 136, 109–115. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Choi, W.G.; Kim, W.J.; Kim, W.K.; Kim, M.J.; Kang, W.H.; Kim, C.M. Antimicrobial constituents of Foeniculum vulgare. Arch. Pharmacal Res. 2002, 25, 154–157. [Google Scholar] [CrossRef]
- Tzeng, T.C.; Lin, Y.L.; Jong, T.T.; Chang, C.M.J. Ethanol modified supercritical fluids extraction of scopoletin and artemisinin from Artemisia annua L. Sep. Purif. Technol. 2007, 56, 18–24. [Google Scholar] [CrossRef]
- Shaw, C.Y.; Chen, C.H.; Hsu, C.C.; Chen, C.C.; Tsai, Y.C. Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother. Res. 2003, 17, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, M.C.; Ferrayoli, C.G.; Palacios, S.M. Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits. J. Agric. Food Chem. 2005, 53, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Wang, L.; Wei, L.J.; Zheng, Y.; Zhang, H.B.; Wang, Y.B.; Cao, P.; Niu, A.; Wang, J.; Dai, Y. Synthesis and antitumor activity of scopoletin derivatives. Lett. Drug Des. Discov. 2012, 9, 397–401. [Google Scholar] [CrossRef]
- Zhang, Y.Q. Studies on Acaricidal Action Mechanism and Bio-Guided Isolation of Bioaetive Substance from Artemisia annua L. Ph.D. Thesis, Southwest University, Chongqing, China, 2008. [Google Scholar]
- Liang, W.; Bai, X.N.; Cheng, J.; Shi, G.L.; Wang, Y.N.; Wang, Z.Q. Isolation and identification of the principal acaricidal components from stellera chamaejasme. Acta Hortic. Sin. 2011, 38, 947–954. [Google Scholar]
- Ding, Z.Q.; Dai, Y.; Hao, H.P.; Pan, R.; Yao, X.J.; Wang, Z.T. Anti-inflammatory effects of scopoletin and underlying mechanisms. Pharm. Bio. 2008, 46, 854–860. [Google Scholar] [CrossRef]
- Jamuna, S.; Karthika, K.; Paulsamy, S.; Thenmozhi, K.; Kathiravan, S.; Venkatesh, R. Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind. Crop. Prod. 2015, 70, 221–230. [Google Scholar] [CrossRef]
- Cassady, J.M.; Ojima, N.; Chang, C.J.; McLaughlin, J.L. An investigation of the antitumor activity of Micromelum integerrimum (Rutaceae). J. Nat. Prod. 1979, 42, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Sung, S.H.; Park, J.H.; Kim, Y.C. Hepatoprotective activity of scopoletin, a constituent of Solanum lyratum. Arch. Pharm. Res. 1998, 21, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Bhakuni, R.S.; Upadhyay, S.; Gaur, R. Insect feeding deterrent and growth inhibitory activities of scopoletin isolated from Artemisia annua against Spilarctia obliqua (Lepidoptera: Noctuidae). Insect Sci. 2011, 18, 189–194. [Google Scholar] [CrossRef]
- Prats, E.; Bazzalo, M.E.; León, A.; Jorrín, J.V. Fungitoxic effect of scopolin and related coumarins on Sclerotinia sclerotiorum. A way to overcome sunflower head rot. Euphytica 2006, 147, 451–460. [Google Scholar] [CrossRef]
- Pérez, F.J.; Nuñez, J.O. Root exudates of wild oats: Allelopathic effect on spring wheat. Phytochemistry 1991, 30, 2199–2202. [Google Scholar] [CrossRef]
- Ezeokonkwo, C.A.; Obidoa, O.; Eze, L.C. Effects of scopoletin and aflatoxin B1 on bovine erythrocyte membrane Na-K-ATPase. Plant Physiol. Commun. 2010, 41, 715–719. [Google Scholar]
- Ezeokonkwo, C.A.; Obidoa, O. Effect of scopoltin on erythrocyte membrane ion motive ATPases. Niger. J. Nat. Prod. Med. 2001, 5, 37–40. [Google Scholar]
- Ojewole, J.A.; Adesina, S.K. Cardiovascular and neuromuscular actions of scopoletin from fruit of Tetrapleura tetraptera. Planta Med. 1983, 49, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.L.; Zhang, Y.Q.; Li, C.X.; Ding, W.; Liu, X.J.; Luo, J.X. Acaricidal toxicity of scopoletin combined with Ca2+ and its influence on Ca2+-ATPase activity in Tetranychus cinnabarinus (Boisduval). Chin. J. Pestic. Sci. 2015, 17, 475–479. [Google Scholar]
- Hou, Q.L.; Luo, J.X.; Zhang, B.C.; Jiang, G.F.; Ding, W.; Zhang, Y.Q. 3D-QSAR and molecular docking studies on the TcPMCA1-mediated detoxification of scopoletin and coumarin derivatives. Int. J. Mol. Sci. 2017, 18, 1380. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Worachartcheewan, A.; Shoombuatong, W.; Songtawee, N.; Simeon, S.; Prachayasittikul, V.; Nantasenamat, C. Computer-aided drug design of bioactive natural products. Curr. Top. Med. Chem. 2015, 15, 1780–1800. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nut. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, anticanceractivity and QSAR study of 1,4-naphthoquinone derivatives. Eur. J. Med. Chem. 2014, 84, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Novel 1,4-naphthoquinone-based sulfonamides: Synthesis, QSAR, anticancer and antimalarial studies. Eur. J. Med. Chem. 2015, 103, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Mandi, P.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, molecular docking, and QSAR study of sulfonamide-based indoles as aromatase inhibitors. Eur. J. Med. Chem. 2018, 143, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shao, Y.H.; Zhi, X.Y.; Huan, Q.; Yu, X.; Yao, X.J.; Xu, H. Semisynthesis and quantitative structure–activity relationship (QSAR) study of some cholesterol-based hydrazone derivatives as insecticidal agents. Bioorg. Med. Chem. Lett. 2013, 23, 4806–4812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nan, X.; Yu, H.T.; Cheng, P.L.; Zhang, Y.; Liu, Y.Q.; Zhang, S.Y.; Hu, G.F.; Liu, H.X.; Chen, A.L. Synthesis, biological activities and structure activity relationships for new avermectin analogues. Eur. J. Med. Chem. 2016, 121, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Du, Y.Z.; Jiang, D.X.; Behnke, C.; Nomura, Y.; Zhorov, B.S.; Dong, K. The receptor site and mechanism of action of sodium channel blocker insecticides. J. Biol. Chem. 2016, 291, 20113–20124. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.W.; Jin, J.; Zhu, J.; Wang, H.F.; Yang, D.R.; Xing, J.H. Discovery of bis-aromatic ring neonicotinoid analogues fixed as cis-configuration: Synthesis, insecticidal activities, and molecular docking studies. Bioorg. Med. Chem. Lett. 2010, 20, 3301–3305. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Yang, J.; Zhou, J.P.; Lu, W.G.; Hu, C.P.; Gu, Z.H.; Huo, J.G.; Wang, X.N.; Gao, P. Synthesis and biological evaluation of scopoletin derivatives. Bioorg. Med. Chem. 2013, 21, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Xue, Z.X.; Wang, N.W. Research and Development of New Pesticides. Chem. Ind. Press 1996, 23–24. [Google Scholar]
- Chen, M.M.; Yang, X.M.; Lai, X.M.; Gao, Y.X. 2D and 3D QSAR models for identifying diphenylpyridylethanamine based inhibitors against cholesteryl ester transfer protein. Bioorg. Med. Chem. Lett. 2015, 25, 4487–4495. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.; Jensen, R.; Heyden, Y.V. QSRR modeling for diverse drugs using different feature selection methods coupled with linear and nonlinear regressions. J. Chromatogr. B 2012, 910, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.J.; Baboli, M.A.; Shahbazikhah, P. QSAR study of heparanase inhibitor activity using artificial neural networks and Levenverg-Marquardt algorithm. Eur. J. Med. Chem. 2008, 43, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.N.; Khan, F.A.K.; Kulkarni, A.A.; Patil, R.H.; Pachpinde, A.M.; Lohar, K.S.; Shinde, D.B. Antileishmanial activity of novel indolyl-coumarin hybrids: Design, synthesis, biological evaluation, molecular docking study and in silico ADME prediction. Bioorg. Med. Chem. Lett. 2016, 26, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Busvine, J.R. Recommended Methods for Measurement of Resistance to Pesticides; Plant Production and Protection 21; FAO: Rome, Italy, 1980; pp. 49–54. [Google Scholar]
- Hu, J.F.; Wang, C.F.; Wang, J.; You, Y.; Chen, F. Monitoring of resistance to spirodiclofen and five other acaricides in Panonychus citri collected from Chinese citrus orchards. Pest Manag. Sci. 2010, 66, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, Q.G.; Li, Y.X.; Tang, L. A new model of flavonoids affinity towards P-glycoprotein: Genetic algorithm-support vector machine with features selected by a modified particle swarm optimization algorithm. Arch. Pharm. Res. 2017, 40, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Bai, F.; Cao, H.; Li, J.Z.; Liu, H.X.; Gramatica, P. A combined quantitative structure-activity relationship research of quinolinone derivatives as androgen receptor antagonists. Comb. Chem. High Throughput Scr. 2015, 18, 834–845. [Google Scholar] [CrossRef]
- Zhou, A.; Hu, J.P.; Wang, L.R.; Zhong, G.C.; Pan, J.; Wu, Z.Y.; Hui, A.l. Combined 3D-QSAR, molecular docking, and molecular dynamics study of tacrine derivatives as potential acetylcholinesterase (AChE) inhibitors of Alzheimer’s disease. J. Mol. Model. 2015, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Geladi, P.; Esbensen, K.; Öhman, J. Multi-way principal components and PLS analysis. J. Chemometr. 1987, 1, 41–56. [Google Scholar] [CrossRef]
- Ao, J.J.; Gao, L.; Yuan, T.; Jiang, G.F. Interaction mechanisms between organic UV filters and bovine serum albumin as determined by comprehensive spectroscopy exploration and molecular dockiong. Chemosphere 2015, 119, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Molinspiration Chemoinformatics, Brastislava, Slovak Republic. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 20 December 2017).
- Zhao, Y.H.; Abraham, M.H.; Le, J.; Hersey, A.; Luscombe, C.N.; Beck, G.; Sherborne, B.; Cooper, I. Rate-Limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4, and 8–37 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).