Fusaproliferin, a Fungal Mycotoxin, Shows Cytotoxicity against Pancreatic Cancer Cell Lines
Abstract
:1. Introduction
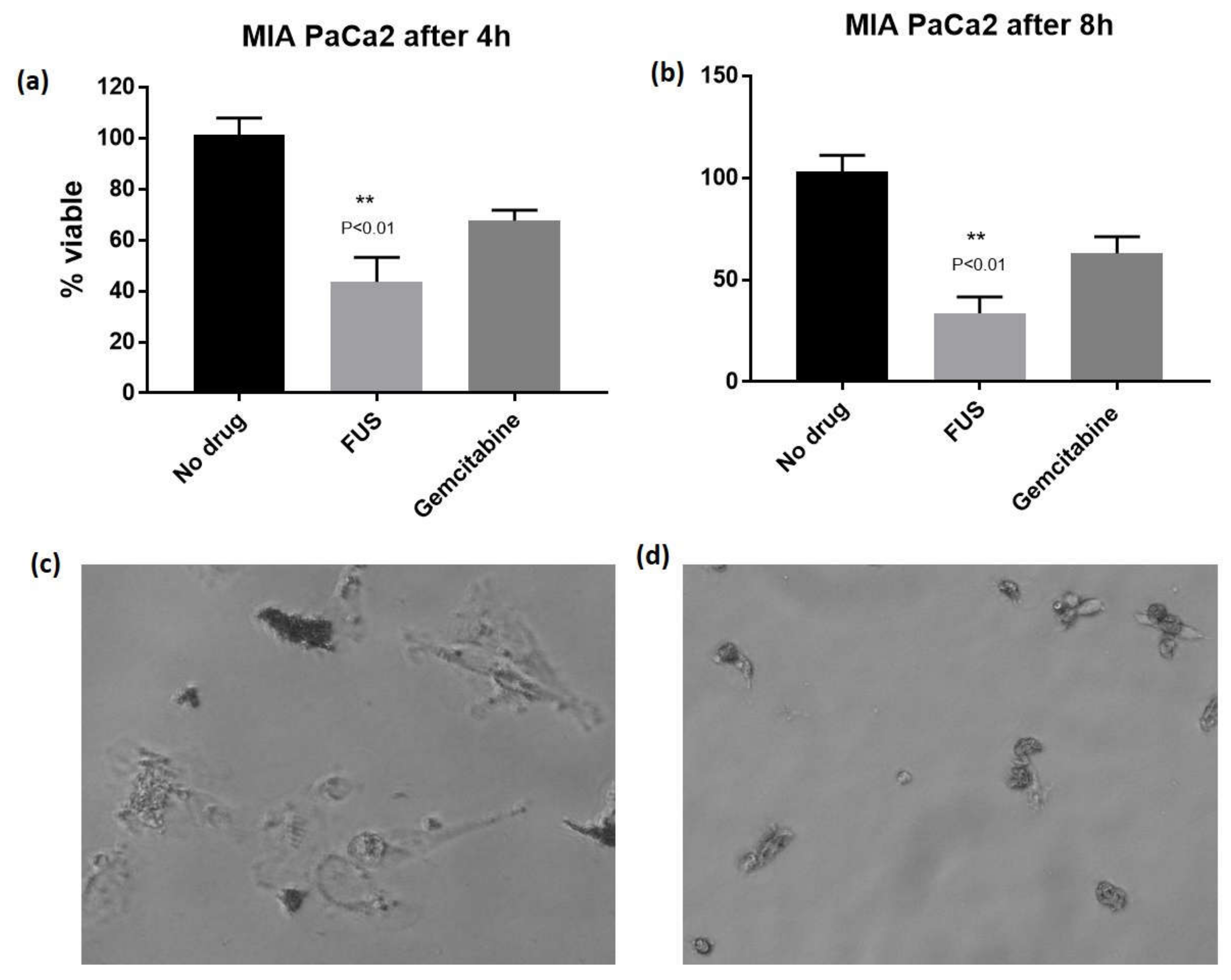
2. Results and Discussion
3. Experimental Section
3.1. Collection and Identification of the Plant Material
3.2. General Experimental Procedures
3.3. Isolation of Fungal Material
3.4. Molecular Identification of the Endophytic Fungus AHPE-4
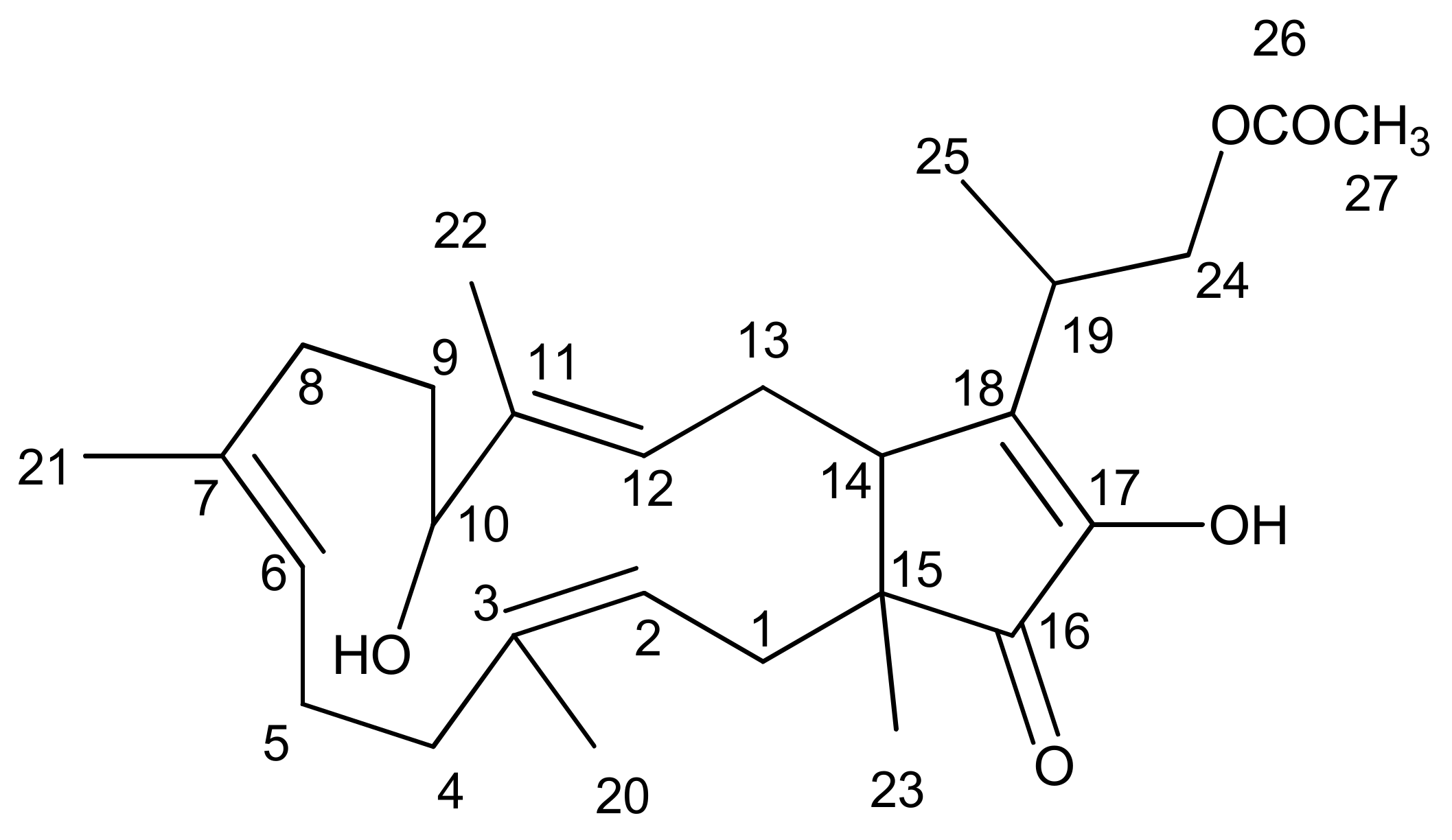
3.5. Extraction of the Fungal Material and Isolation of FUS
Fusaproliferin
3.6. Bioassays
3.6.1. Cell Culture
3.6.2. MTT Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Vervenne, W.L.; Bennouna, J.; Humblet, Y.; Gill, S.; Van Laethem, J.-L.; Verslype, C.; Scheithauer, W.; Shang, A.; Cosaert, J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009, 27, 2231–2237. [Google Scholar] [CrossRef]
- Siriwardena, A.K.; Siriwardena, A.M. Pancreatic cancer. BMJ Br. Med. J. 2014, 349. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Jiang, J.Y.; Liang, M.; Fang, Y.; Yeung, M.S.; Sung, J.J.Y. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci. Rep. 2017, 7, 3165. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.Y.; Lu, R.; Wang, J.H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Disc. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef]
- Nascimento, A.M.d.; Conti, R.; Turatti, I.C.; Cavalcanti, B.C.; Costa-Lotufo, L.V.; Pessoa, C.; de Moraes, M.O.; Manfrim, V.; Toledo, J.S.; Cruz, A.K. Bioactive extracts and chemical constituents of two endophytic strains of Fusarium oxysporum. Rev. Bras. Farmacogn. 2012, 22, 1276–1281. [Google Scholar] [CrossRef]
- Cui, Y.; Yi, D.; Bai, X.; Sun, B.; Zhao, Y.; Zhang, Y. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 2012, 83, 913–920. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Sohrab, M.H.; Rony, S.R.; Tareq, F.S.; Hasan, C.M.; Mazid, M.A. Cytotoxic and antibacterial naphthoquinones from an endophytic fungus, Cladosporium sp. Toxicol. Rep. 2016, 3, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.S.; Sohrab, M.H.; Rony, S.R.; Sharmin, S.; Begum, M.N.; Rana, M.S.; Hasan, C.M. Identification and bioactive potential of endophytic fungi from Monochoria hastata (L.) Solms. Bangladesh J. Bot. 2016, 45, 187–193. [Google Scholar]
- Chowdhury, N.S.; Sohrab, M.H.; Rana, M.S.; Hasan, C.M.; Jamshidi, S.; Rahman, K.M. Cytotoxic naphthoquinone and azaanthraquinone derivatives from an endophytic Fusarium solani. J. Nat. Prod. 2017, 80, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afroz, F.; Begum, M.N.; Rony, S.R.; Sharmin, S.; Moni, F.; Hasan, C.M.; Shaha, K.; Sohrab, M.H. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthoquinone and aza-anthraquinone derivatives. Toxicol. Rep. 2018, 5, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Kamisuki, S.; Chia, P.T.; Kuriyama, I.; Mizushina, Y.; Sugawara, F. Bioactive dihydronaphthoquinone derivatives from Fusarium solani. J. Nat. Prod. 2014, 77, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Shweta, S.; Zuehlke, S.; Ramesha, B.T.; Priti, V.; Kumar, M.P.; Ravikanth, G.; Spiteller, M.; Vasudeva, R.; Shaanker, U.R. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 2010, 71, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, B.; Das, P.; Surendranath, K.; Karande, A.A.; Jayabaskaran, C. Production of paclitaxel by Fusarium solani isolated from Taxus celebica. J. Biosci. 2008, 33, 259–267. [Google Scholar] [CrossRef]
- Kosmidis, C.; Denning, D.W. Opportunistic and Systemic Fungi. In Infectious Diseases, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1681–1709.e3. [Google Scholar]
- Randazzo, G.; Foglianoa, V.; Ritieni, A.; Rossin, L.M.E.; Scarallo, A.; Segred, A.L. Proliferin, a new sesterterpene from Fusarium proliferatum. Tetrahedron 1993, 49, 10883–10896. [Google Scholar] [CrossRef]
- Ritieni, A.; Fogliano, V.; Randazzo, G.; Scarallo, A.; Logrieco, A.; Moretti, A.; Manndina, L.; Bottalico, A. Isolation and characterization of fusaproliferin, a new toxic metabolite from Fusarium proliferatum. Nat. Toxins 1995, 3, 17–20. [Google Scholar] [CrossRef]
- Santini, A.; Ritieni, A.; Fogliano, V.; Randazzo, G.; Mannina, L.; Logrieco, A.; Benedetti, E. Structure and absolute stereochemistry of fusaproliferin, a toxic metabolite from Fusarium proliferatum. J. Nat. Prod. 1996, 59, 109–112. [Google Scholar] [CrossRef]
- Fotso, J.; Leslie, J.F.; Smith, S.J. Production of beauvericin, moniliformin, fusaproliferin, and fumonisins B1, B2, and B3 by fifteen ex-type strains of Fusarium Species. Appl. Environ. Microbiol. 2002, 68, 5195–5197. [Google Scholar] [CrossRef] [PubMed]
- Ritieni, A.; Monti, S.M.; Moretti, A.; Logrieco, A.; Gallo, A.; Ferracane, R.; Fogliano, V. Stability of fusaproliferin, a mycotoxin from Fusarium spp. J. Sci. Food Agric. 1999, 79, 1676–1680. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Fornelli, F.; Fogliano, V.; Ritieni, A.; Caiaffa, M.F.; Randazzo, G.; Bottalico, A.; Macchia, L. Fusaproliferin production by Fusarium subglutinans and its toxicity to Artemia salina, SF-9 insect cells, and IARC/LCL 171 human B lymphocytes. Appl. Environ. Microbiol. 1996, 62, 3378–3384. [Google Scholar] [PubMed]
- Ritieni, A.; Monti, S.M.; Randazzo, G.; Logrieco, A.; Moretti, A.; Peluso, G.; Ferracane, R.; Fogliano, V. Teratogenic effects of fusaproliferin on chicken embryos. J. Agric. Food Chem. 1997, 45, 3039–3043. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. However, the compounds can be extracted again from the natural source. |
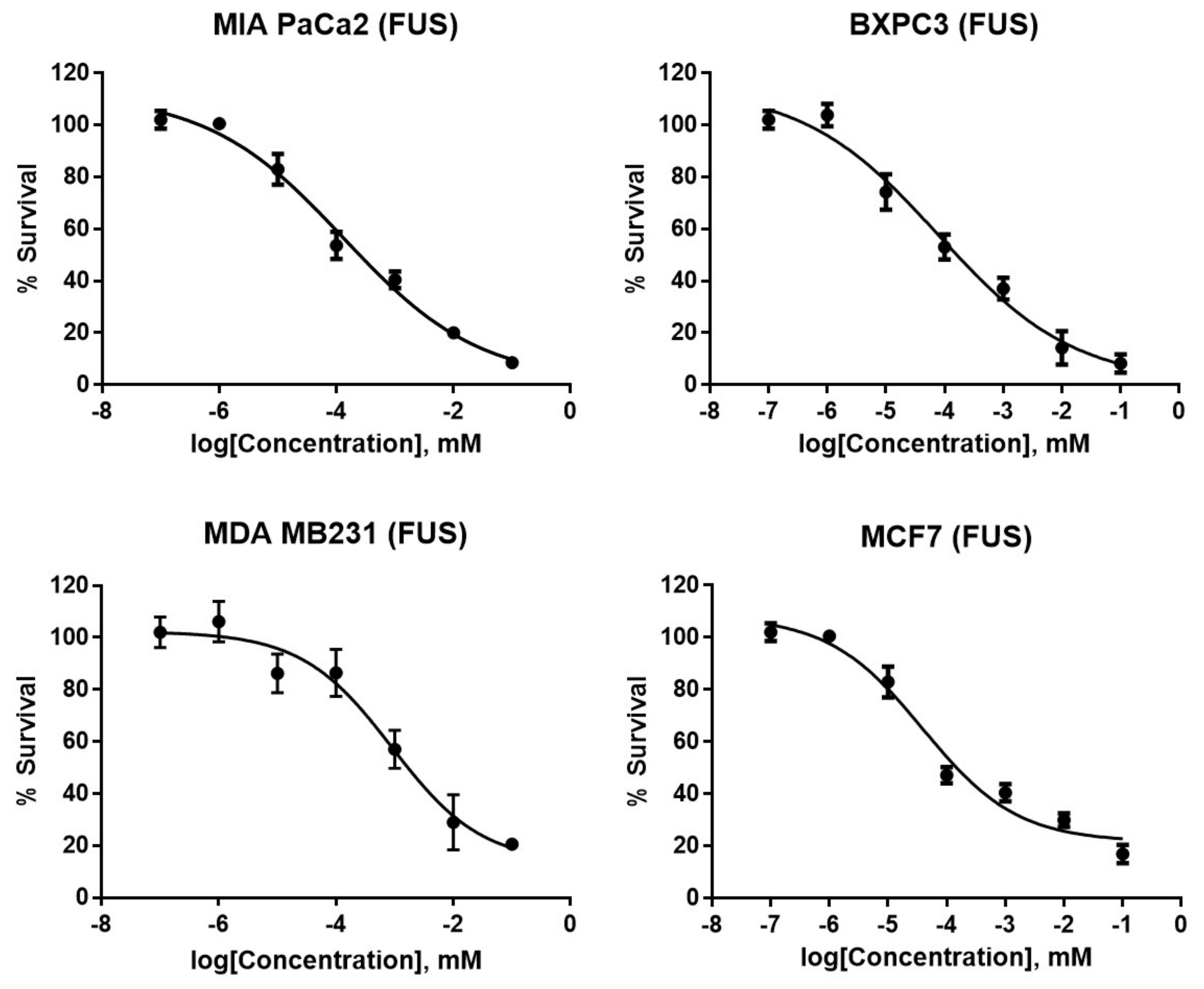
| Compound/Standard | MIA PaCa 2 (Pancreatic) | BXPC3 (Pancreatic) | MDA MB 231 (Breast) | MCF7 (Breast) | WI 38 (Lung Fibroblast) |
|---|---|---|---|---|---|
| FUS | 0.13 ± 0.09 | 0.76 ± 0.24 | 1.9 ± 0.32 | 3.9 ± 0.75 | 18 ± 0.66 |
| Gemcitabine | 7.6 ± 0.66 | 2.2 ± 0.43 | NT | NT | NT |
| Doxorubicin | NT | NT | 0.06 ± 0.03 | 0.02 ± 0.018 | NT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, N.; Hasan, C.M.; Rana, M.S.; Varsha, A.; Sohrab, M.H.; Rahman, K.M. Fusaproliferin, a Fungal Mycotoxin, Shows Cytotoxicity against Pancreatic Cancer Cell Lines. Molecules 2018, 23, 3288. https://doi.org/10.3390/molecules23123288
Hoque N, Hasan CM, Rana MS, Varsha A, Sohrab MH, Rahman KM. Fusaproliferin, a Fungal Mycotoxin, Shows Cytotoxicity against Pancreatic Cancer Cell Lines. Molecules. 2018; 23(12):3288. https://doi.org/10.3390/molecules23123288
Chicago/Turabian StyleHoque, Nazia, Choudhury Mahmood Hasan, Md. Sohel Rana, Amrit Varsha, Md. Hossain Sohrab, and Khondaker Miraz Rahman. 2018. "Fusaproliferin, a Fungal Mycotoxin, Shows Cytotoxicity against Pancreatic Cancer Cell Lines" Molecules 23, no. 12: 3288. https://doi.org/10.3390/molecules23123288






