Field Efficacy of Cordyceps javanica, White Oil and Spinetoram for the Management of the Asian Citrus Psyllid, Diaphorina citri
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Treatments
2.2. Spore Deposition
2.3. AsCP and Natural Enemy Abundance
2.4. Cordyceps javanica Field Persistence and Pathogenicity
2.5. Statistical Analyses
3. Results
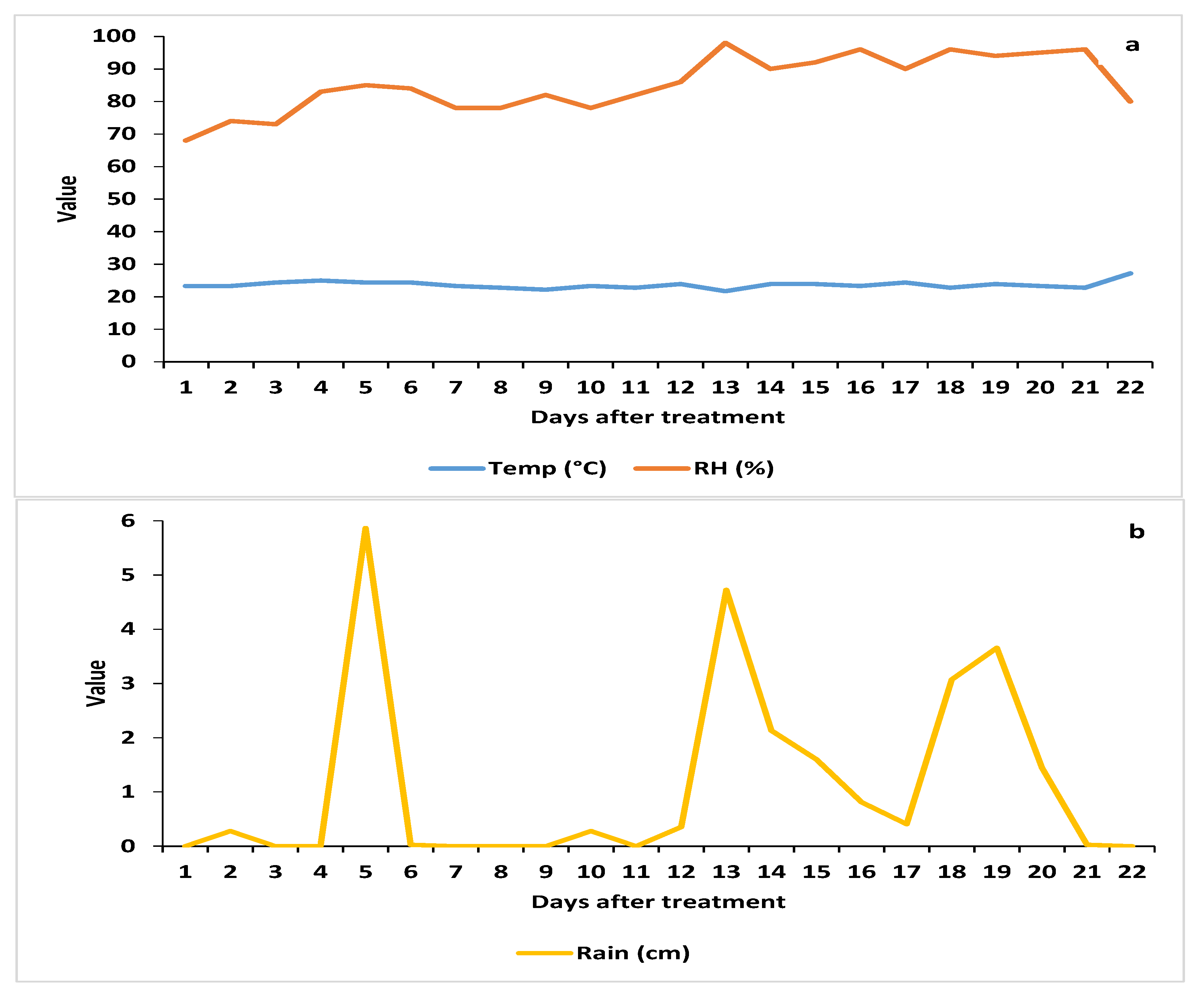
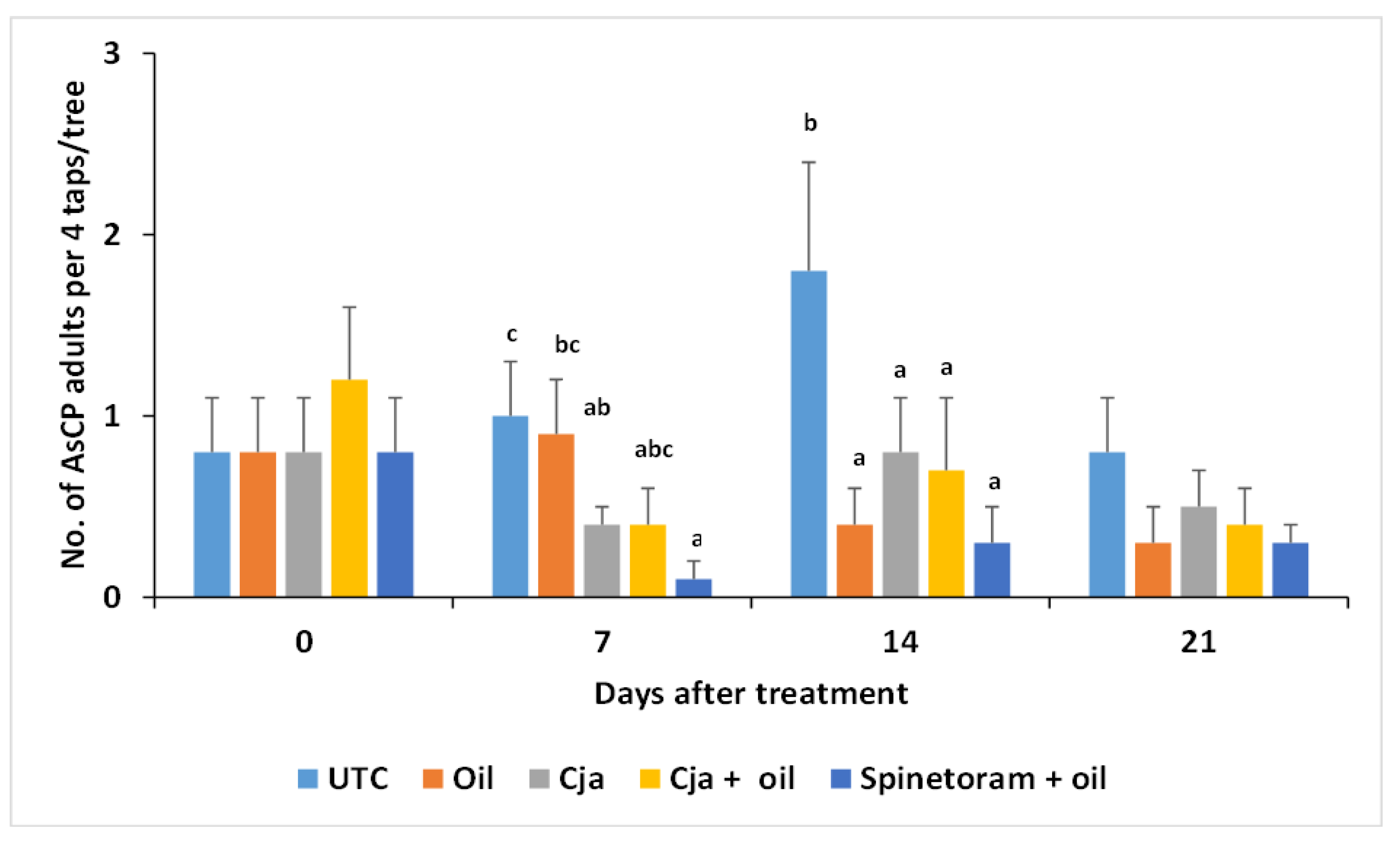
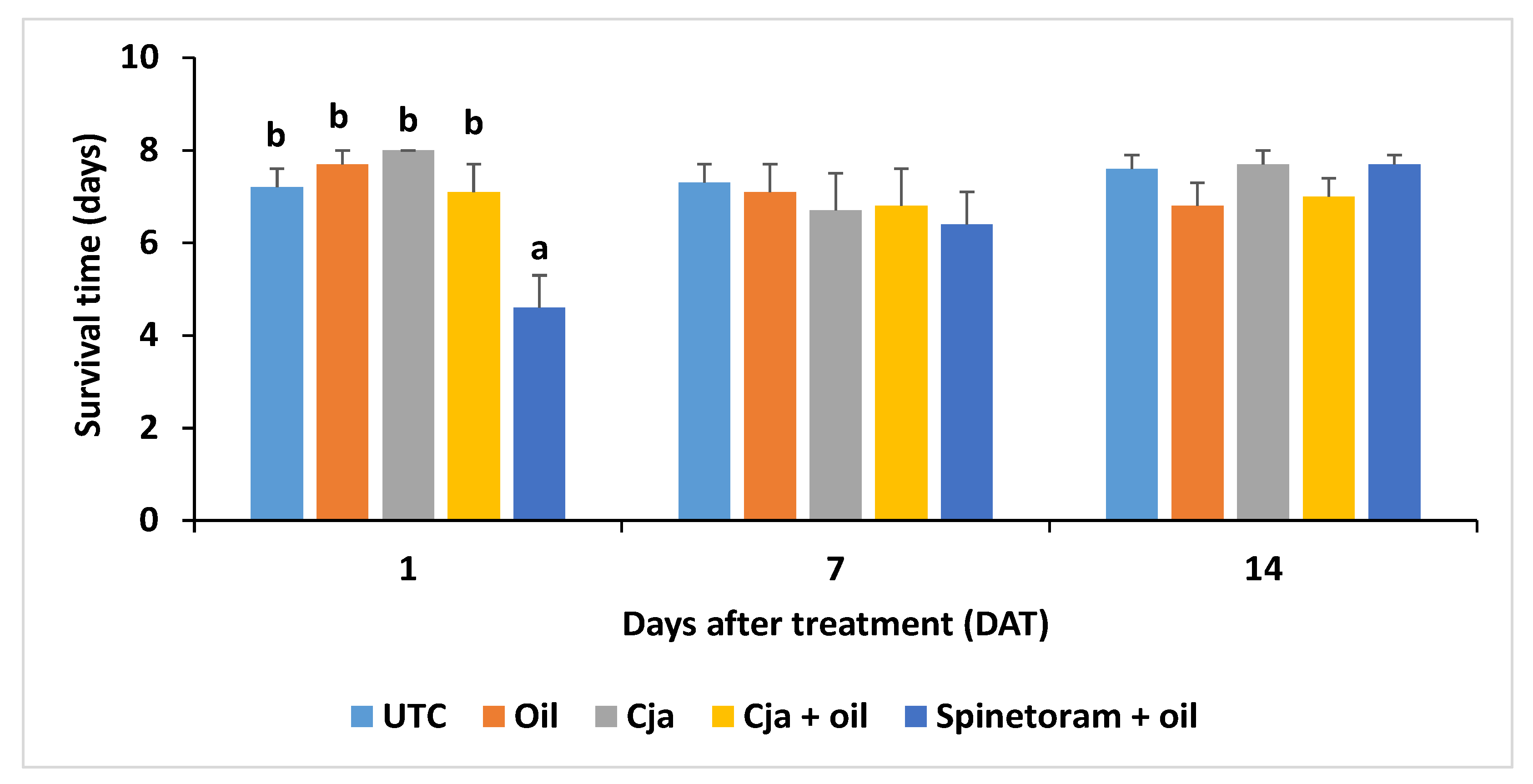
3.1. Trial 1—2018
3.1.1. Weather Parameters
3.1.2. Spore Deposition
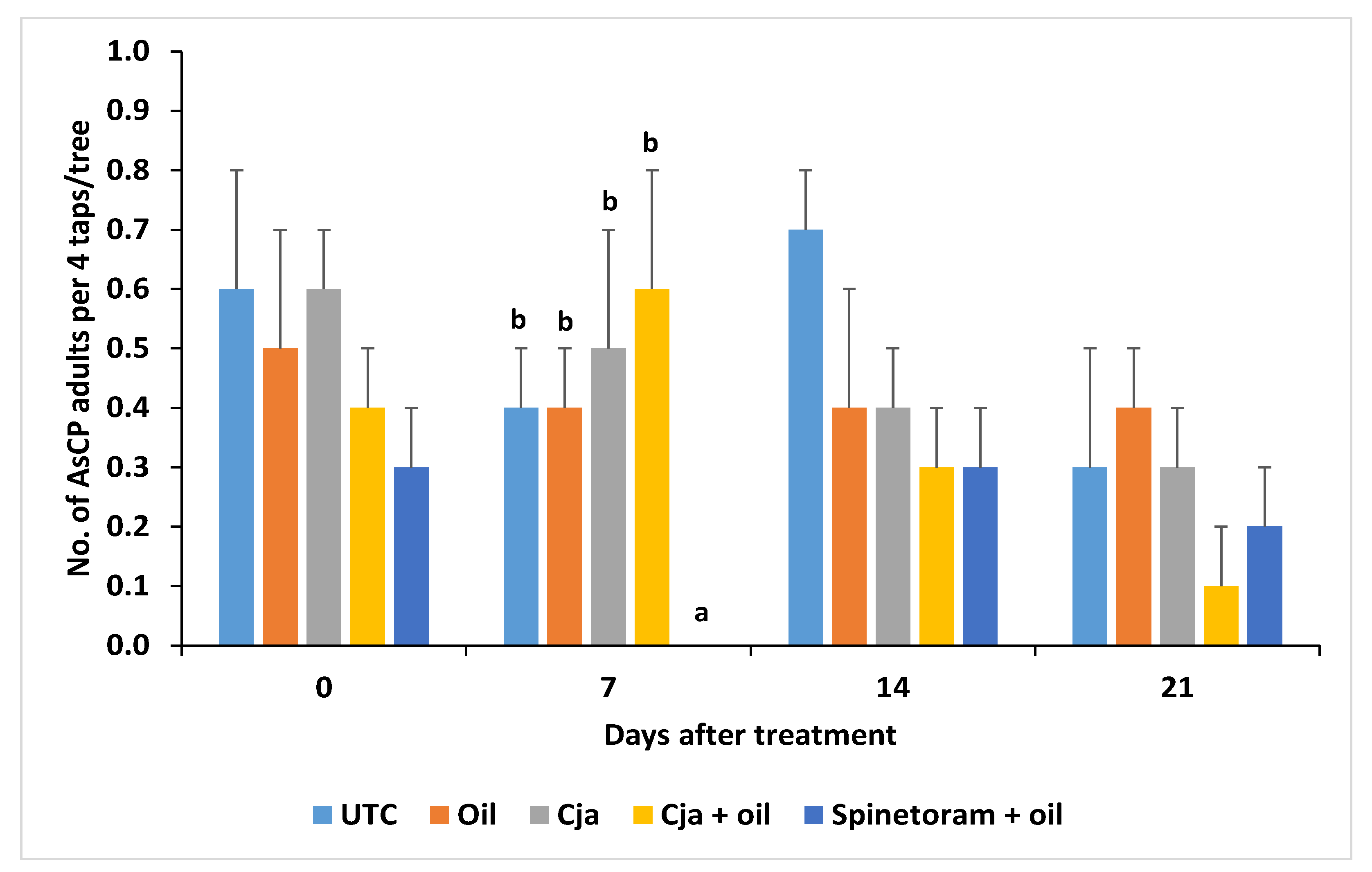
3.1.3. AsCP and Natural Enemy Abundance
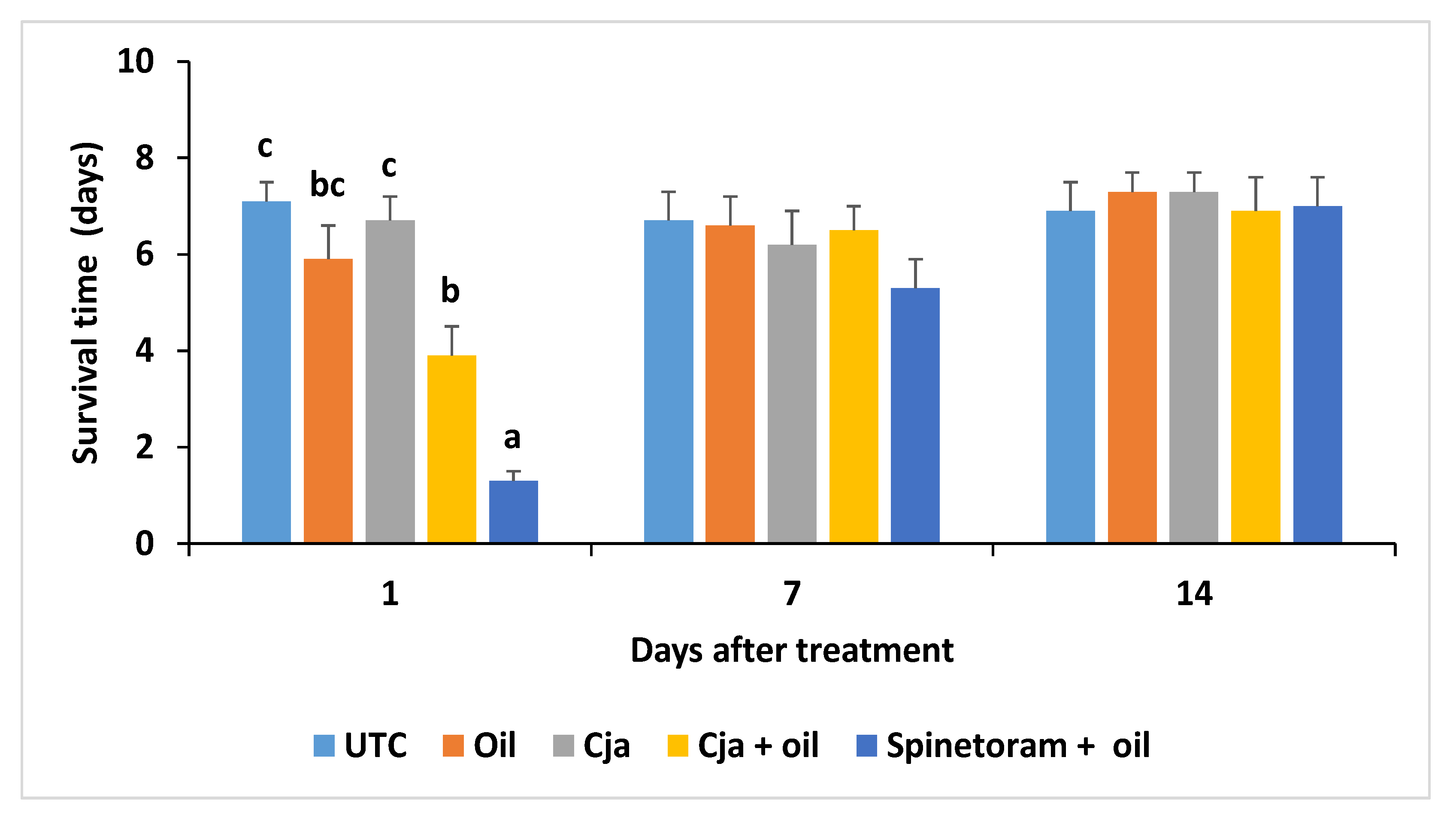
3.1.4. Cordyceps javanica Field Persistence and Pathogenicity
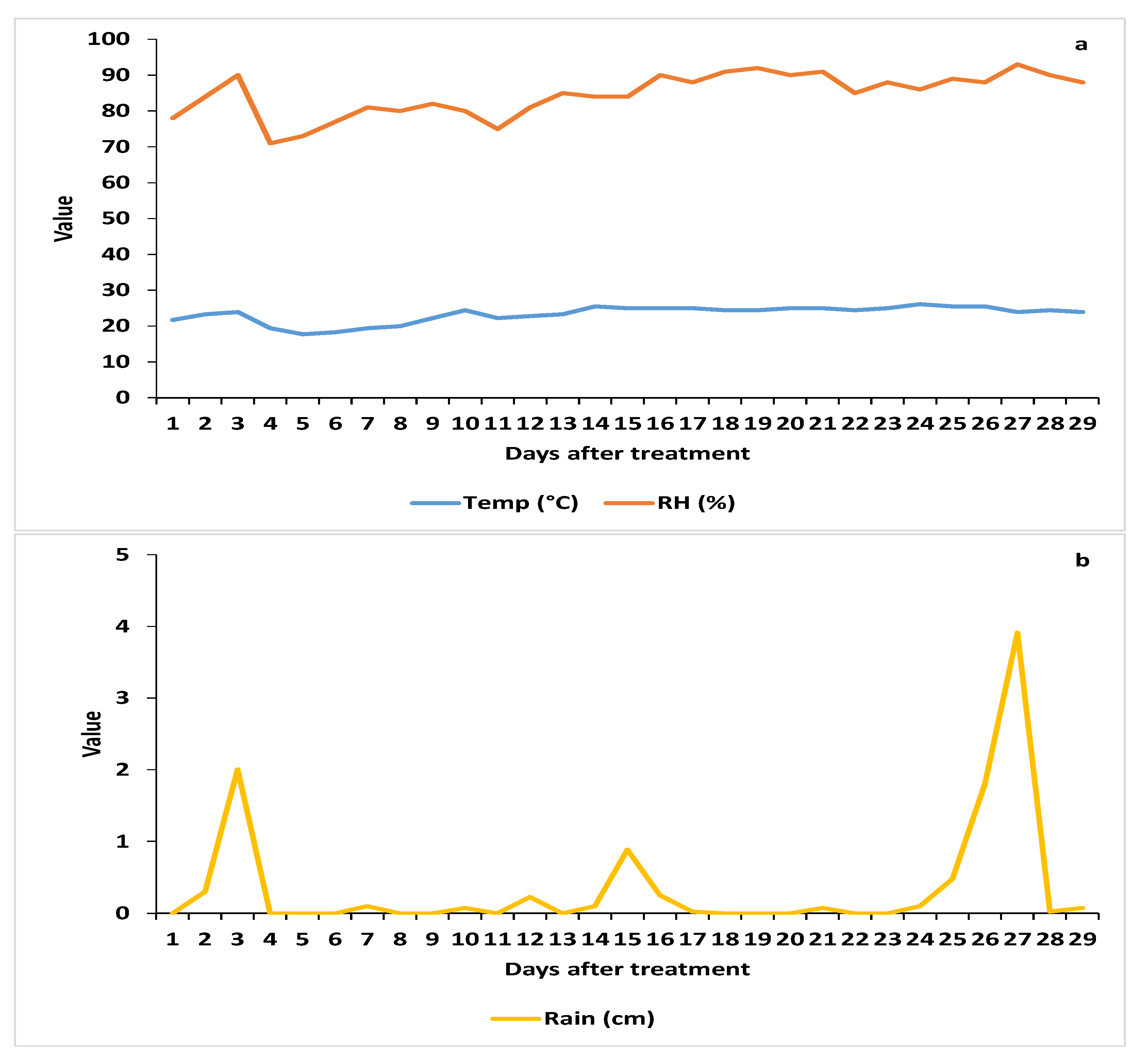
3.2. Trial 2—2019
3.2.1. Weather Parameters
3.2.2. Spore Deposition
3.2.3. AsCP and Natural Enemy Abundance
3.2.4. Cordyceps javanica Field Persistence and Pathogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, D.G.; Richardson, M.L.; Ammar, E.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging century-old disease of citrus. J. Plant. Pathol. 2006, 88, 7–37. [Google Scholar]
- Manjunath, K.L.; Halbert, S.E.; Ramadugu, C.; Webb, S.; Lee, R.F. Detection of ‘Candidatus’ Liberibacter asiaticus’ in Diaphorina citri and its importance in the management of citrus huanglongbing in Florida. Phytopathology 2008, 98, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottwald, T.R. Current epidemiological understanding of citrus huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [Green Version]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, D.; Moss, C.B.; Van Bruggen, A.H.C. Estimating citrus production loss due to citrus huanglongbing in Florida. In Proceedings of the Southern Agricultural Economics Association Annual Meeting, San Antonio, TX, USA, 6–9 February 2016; Available online: https://ageconsearch.umn.edu/bitstream/230093/2/Estimating%20CitrusFinal.pdf (accessed on 20 January 2021).
- Rogers, M.E. General pest management considerations. Citrus Ind. 2008, 89, 12–15. [Google Scholar]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. PLoS ONE 2014, 12, e11231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest. Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef]
- Tiwari, S.; Lewis-Rosenblum, H.; Pelz-Stelinski, K.; Stelinski, L.L. Incidence of Candidatus Liberibacter asiaticus infection in abandoned citrus occurring in proximity to commercially managed groves. J. Econ. Entomol. 2010, 103, 1972–1978. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest. Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Michaud, J.P. Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol. Control 2004, 29, 260–269. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Stansly, P.A. Exclusion techniques reveal significant biotic mortality suffered by Asian citrus psyllid Diaphorina citri (Hemiptera: Psyllidae) populations in Florida citrus. Biol. Control 2009, 50, 129–136. [Google Scholar] [CrossRef]
- Hall, D.G.; Nguyen, R. Toxicity of pesticides to Tamarixia radiata, a parasitoid of the Asian citrus psyllid. BioControl 2010, 55, 601–611. [Google Scholar] [CrossRef]
- Nayga, R.M. Sociodemographic influences on consumer concern for food safety: The case of irradiation, antibiotics, hormones, and pesticides. Rev. Agric. Econ. 1996, 18, 467–475. [Google Scholar] [CrossRef]
- Rana, J.; Paul, J. Consumer behavior and purchase intention for organic food: A review and research agenda. J. Retail. Consum. Serv. 2017, 38, 157–165. [Google Scholar] [CrossRef]
- Swanberg, J.E.; Nichols, H.M.; Clouser, J.M.; Check, P.; Edwards, L.; Bush, A.M.; Padilla, Y.; Betz, G. A systematic review of community health workers’ role in occupational safety and health research. J. Immigr. Minor. Health 2018, 20, 1516–1531. [Google Scholar] [CrossRef]
- Meyer, J.M.; Hoy, M.A.; Boucias, D.G. Isolation and characterization of an Isaria fumosorosea isolate infecting the Asian citrus psyllid in Florida. J. Inverter. Pathol. 2008, 99, 96–102. [Google Scholar] [CrossRef]
- Avery, P.B.; Hunter, W.B.; Hall, D.G.; Jackson, M.A.; Powell, C.A.; Rogers, M.E. Diaphorina citri (Hemiptera: Psyllidae) infection and dissemination of the entomopathogenic fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Fla. Entomol. 2009, 92, 608–618. [Google Scholar] [CrossRef]
- Avery, P.B.; Wekesa, V.W.; Hunter, W.B.; Hall, D.G.; McKenzie, C.L.; Osborne, L.S.; Powell, C.A.; Rogers, M.E. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol. Sci. Technol. 2011, 21, 1065–1078. [Google Scholar] [CrossRef]
- Osborne, L.S.; Landa, Z. Biological control of whiteflies with entomopathogenic fungi. Fla. Entomol. 1992, 75, 456–471. [Google Scholar] [CrossRef]
- Cabanillas, H.E.; de León, J.H.; Humber, R.A.; Murray, K.D.; Jones, W.A. Isaria poprawskii sp. nov. (Hypocreales: Cordycipitaceae), a new entomopathogenic fungus from Texas affecting sweet potato whitefly. Mycoscience 2013, 54, 158–169. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Mascarin, G.M.; Romagnoli, E.M.; Jackson, M.A. Rapid discrimination of Isaria javanica and Isaria poprawskii from Isaria spp. using high resolution DNA melting assays. J. Invertebr. Pathol. 2017, 150, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Avery, P.B.; Pick, D.; Powell, C.A. Broad spectrum potential of Isaria fumosorosea on insect pests of citrus. Fla. Entomol. 2011, 94, 1051–1054. [Google Scholar] [CrossRef]
- Stauderman, K.; Avery, P.B.; Aristizábal, L.F.; Arthurs, S.P. Evaluation of Isaria fumosorosea (Hypocreales: Cordycipitaceae) for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol. Sci. Technol. 2012, 22, 747–761. [Google Scholar] [CrossRef]
- Avery, P.B.; Pick, D.A.; Aristizábal, L.; Kerrigan, J.; Powell, C.A.; Rogers, M.E.; Arthurs, S.P. Compatibility of Isaria fumosorosea (Hypocreales: Cordycipitaceae) blastospores with agricultural chemicals used for management of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2013, 4, 694–711. [Google Scholar] [CrossRef]
- Kumar, V.; Avery, P.B.; Ahmed, J.; Cave, R.D.; McKenzie, C.L.; Osborne, L.S. Compatibility and efficacy of Isaria fumosorosea with horticultural oils for mitigation of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2017, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Sterk, G.; Bolckmans, K.; de Jonghe, R.; de Wael, L.; Vermeulen, J. Side-effects of the microbial insecticide PreFeRal WG (Paecilomyces fumosoroseus, strain Apopka 97) on Bombus terrestris. Meded. Fac. Landbouwkd. Toegep. Biol. Wet. Univ. Gent 1995, 60, 713–717. [Google Scholar]
- Sterk, G.; Bolckmans, K.; van de Veire, M.; Sels, B.; Stepman, W. Side-effects of the microbial insecticide PreFeRal WG (Paecilomyces fumosoroseus, strain Apopka 97) on different species of beneficial arthropods. Meded. Fac. Landbouwkd. Toegep. Biol. Wet. Univ. Gent 1995, 60, 719–724. [Google Scholar]
- Alma, C.R.; Goettel, M.S.; Roitberg, B.D.; Gillespie, D.R. Combined effects of the entomopathogenic fungus, Paecilomyces fumosoroseus Apopka-97, and the generalist predator, Dicyphus hesperus, on whitefly populations. BioControl 2007, 52, 669–681. [Google Scholar] [CrossRef]
- Sánchez Barahona, C.F.; Threlkeld, B.S.; Avery, P.B.; Francis, A.W.; Cave, R.D. Compatibility and efficacy of the lady beetle Thalassa montezumae and the entomopathogenic fungus Isaria fumosorosea for biological control of the green croton scale: Laboratory and greenhouse investigations. Arthropod-Plant Interact 2018, 12, 715–723. [Google Scholar] [CrossRef]
- Avery, P.B.; Kumar, V.; Skvarch, E.A.; Mannion, C.M.; Powell, C.A.; McKenzie, C.L.; Osborne, L. S An ecological assessment of Isaria fumosorosea applications compared to a neonicotinoid treatment for regulating invasive ficus whitefly. J. Fungi 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, J.A.; Rogers, M.E.; Hall, D.G.; Stansly, P.A. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J. Econ. Entomol. 2009, 102, 247–256. [Google Scholar] [CrossRef]
- Chow, A.; Dunlap, C.A.; Jackson, M.A.; Flores, D.; Patt, J.M.; Sétamou, M. Oviposition behavior and survival of Tamarixia radiata (Hymenoptera: Eulophidae), an ectoparasitoid of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), on hosts exposed to an entomopathogenic fungus, Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. J. Econ. Entomol. 2016, 109, 1995–2005. [Google Scholar]
- Chen, X.; Triana, M.; Stansly, P.A. Optimizing production of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea). Biol. Control 2017, 105, 13–18. [Google Scholar] [CrossRef]
- Chow, A.; Dunlap, C.A.; Jackson, M.A.; Avery, P.B.; Patt, J.M.; Sétamou, M. Field efficacy of autodissemination and foliar sprays of an entomopathogenic fungus, Isaria fumosorosea (Hypocreales: Cordycipitaceae), for control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), on residential citrus. J. Econ. Entomol. 2018, 111, 2089–2100. [Google Scholar] [CrossRef]
- Ibarra-Cortés, K.H.; González-Hernández, H.; Guzmán-Franco, A.W.; Ortega-Arenas, L.D.; Villanueva-Jiménez, J.A.; Robles-Bermúdez, A. Interactions between entomopathogenic fungi and Tamarixia radiata (Hymenoptera: Eulophidae) in Diaphorina citri (Hemiptera: Liviidae) populations under laboratory conditions. J. Pest. Sci. 2018, 91, 373–384. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Qureshi, J.A.; Stelinski, L.L.; Stansly, P.A. Florida Citrus Production Guide: Asian Citrus Psyllid; EDIS Publication CG097; Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. 2019. Available online: https://edis.ifas.ufl.edu/cg097 (accessed on 28 February 2021).
- Knapp, J.L.; Oswalt, T.W. Florida Citrus Integrated Pest and Crop Management Handbook; SP-14; Florida Cooperative Extension Service: Gainesville, FL, USA, 1983; pp. 6–83. [Google Scholar]
- Monzo, C.; Stansly, P.A. Economic injury levels for Asian citrus psyllid control in process oranges from mature trees with high incidence of huanglongbing. PLoS ONE 2017, 12, e0175333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, C.; Fargues, J.; Lacey, L.A. Intraspecific variability of Paecilomyces fumosoroseus: Effect of temperature on vegetative growth. J. Inverteb. Pathol. 1997, 70, 18–26. [Google Scholar] [CrossRef]
- Vega, F.E.; Jackson, M.A.; McGuire, M.R. Germination of conidia and blastospores of Paecilomyces fumosoroseus on the cuticle of the silverleaf whitefly, Bemisia argentifolii. Mycopathologia 1999, 147, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Ausique, J.J.S.; D’Alessandro, C.P.; Conceschi, M.R.; Mascarin, G.M.; Júnior, I.D. Efficacy of entomopathogenic fungi against adult Diaphorina citri from laboratory to field applications. J. Pest. Sci. 2017, 90, 947–960. [Google Scholar] [CrossRef]
- Smits, N.; Fargues, J.; Rougier, M. Modelling the persistence of quiescent conidia of the entomopathogenic hyphomycete Paecilomyces fumosoroseus exposed to solar radiation. Biocontrol. Sci. Technol. 1997, 7, 365–376. [Google Scholar] [CrossRef]
- Hoy, M.A.; Singh, R.; Rogers, M.E. Evaluations of a novel isolate of Isaria fumosorosea for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla. Entomol. 2010, 93, 24–32. [Google Scholar] [CrossRef]
- Lezema-Gutiérrez, R.; Molina-Ochoa, J.; Chávez-Flores, O.; Ángel-Sahagun, C.A.; Skoda, S.R.; Reyes-Martínez, G.; Barba-Reynoso, M.; Rebolledo-Domínguez, O.; Ruíz-Aguilar, G.M.L.; Foster, J.E. Use of the entomopathogenic fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri (Hemiptera: Psyllidae) in Persian lime under field conditions. Int. J. Trop. Insect. Sci. 2012, 32, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Casique-Valdés, R.; Sánchez-Lara, B.M.; Ek-Mass, J.; Hernández-Guerra, C.; Bidochka, M.; Guízar-Guzmán, L.; López-Arroyo, J.I.; Sánchez-Peña, S.R. Field trial of aqueous and emulsion preparations of entomopathogenic fungi against the Asian citrus psyllid (Hemiptera: Liividae) in a lime orchard in Mexico. J. Entomol. Sci. 2015, 50, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Fiaz, M.; Afzal, M.; Majeed, M.Z. Synergistic action of Isaria fumosorosea Wize (Hypocreales: Cordycipitaceae) and spirotetramat against Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae) under field conditions. Pak. J. Agric. Res. 2018, 31, 194–201. [Google Scholar] [CrossRef]
- Ullah, M.I.; Arshad, M.; Abdullah, A.; Khalid, S.; Iftikhar, Y.; Zahid, S.M.A. Use of the entomopathogenic fungi Beauveria bassiana (Hyphomycetes: Moniliales) and Isaria fumosorosea (Hypocreales: Cordycipitaceae) to control Diaphorina citri Kuwayama (Hemiptera: Liviidae) under laboratory and semi-field conditions. Egypt J. Biol. Pest. Co. 2018, 28, 1–5. [Google Scholar] [CrossRef]
- Moran, P.J.; Patt, J.M.; Cabanillas, H.E.; Adamczyk, J.L.; Jackson, M.A.; Dunlap, C.A.; Hunter, W.B.; Avery, P.B. Localized autoinoculation and dissemination of Isaria fumosorosea for control of the Asian citrus psyllid in South Texas. Subtrop. Plant Sci. 2011, 63, 23–35. [Google Scholar]
- Patt, J.M.; Chow, A.; Meikle, W.G.; Garcia, C.; Jackson, M.A.; Flores, D.; Sétamou, M.; Dunlap, C.A.; Avery, P.B.; Hunter, W.B.; et al. Efficacy of an autodisseminator of an entomopathogenic fungus, Isaria fumosorosea, to suppress Asian citrus psyllid, Diaphorina citri, under greenhouse conditions. Biol. Control 2015, 88, 37–45. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Biresaw, G.; Jackson, M.A. Hydrophobic and electrostatic cell surface properties of blastospores of the entomopathogenic fungus Paecilomyces fumosoroseus. Collids. Surf. B Biointerfaces 2005, 46, 261–266. [Google Scholar] [CrossRef]
| Lady Beetles a | Parasitoid a | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Ha-a | Cc-l | Cc-a | Cs-l | Cs-a | Tr-a | Total NE b |
| Oil | 1 | 1 | |||||
| Cja c | 1 | 1 | 2 | ||||
| Cja + oil | 1 | 1 | 2 | ||||
| Spinetoram + oil | 1 | 1 | |||||
| Untreated control | 1 | 1 | |||||
| Lady Beetles a | Lacewing a | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Ha-a | Cc-l | Cc-a | Cs-l | Cs-a | Ov-a | lw-l | Total NE b |
| Oil | 1 | 1 | 2 | |||||
| Cja c | 2 | 3 | 2 | 1 | 1 | 9 | ||
| Cja + oil | 2 | 1 | 1 | 4 | ||||
| Spinetoram + oil | 1 | 2 | 3 | |||||
| Untreated control | 1 | 1 | 3 | 5 | 1 | 8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avery, P.B.; Duren, E.B.; Qureshi, J.A.; Adair, R.C., Jr.; Adair, M.M.; Cave, R.D. Field Efficacy of Cordyceps javanica, White Oil and Spinetoram for the Management of the Asian Citrus Psyllid, Diaphorina citri. Insects 2021, 12, 824. https://doi.org/10.3390/insects12090824
Avery PB, Duren EB, Qureshi JA, Adair RC Jr., Adair MM, Cave RD. Field Efficacy of Cordyceps javanica, White Oil and Spinetoram for the Management of the Asian Citrus Psyllid, Diaphorina citri. Insects. 2021; 12(9):824. https://doi.org/10.3390/insects12090824
Chicago/Turabian StyleAvery, Pasco B., Emily B. Duren, Jawwad A. Qureshi, Robert C. Adair, Jr., Matthew M. Adair, and Ronald D. Cave. 2021. "Field Efficacy of Cordyceps javanica, White Oil and Spinetoram for the Management of the Asian Citrus Psyllid, Diaphorina citri" Insects 12, no. 9: 824. https://doi.org/10.3390/insects12090824







