Internal Coating of Ureteral Stents with Chemical Vapor Deposition of Parylene
Abstract
:1. Introduction
- Deposition of PPX on inorganic surfaces (metals, glass);
- Deposition of PPX on the internal surface of ureteral stents (here: polyurethane).
2. Experimental
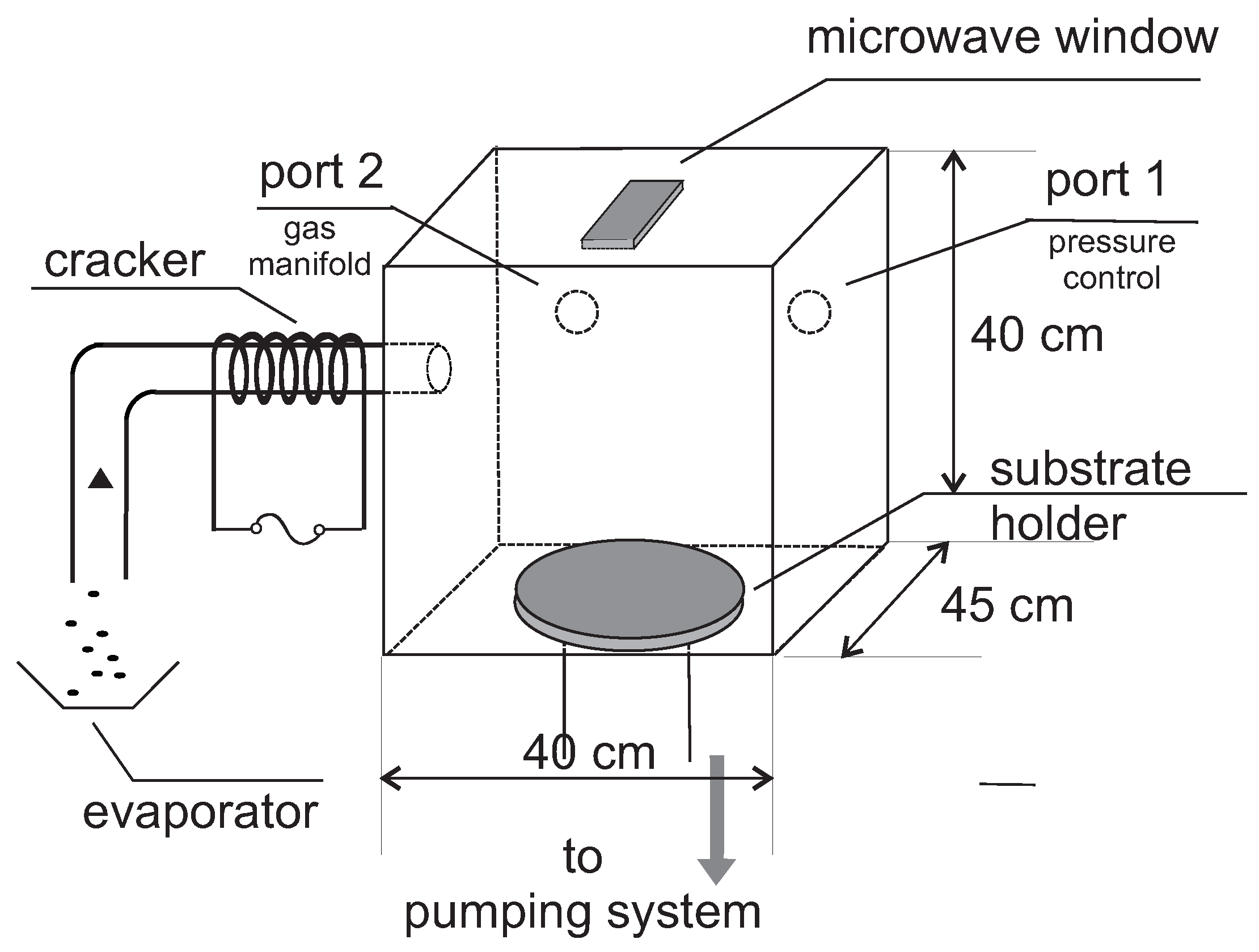
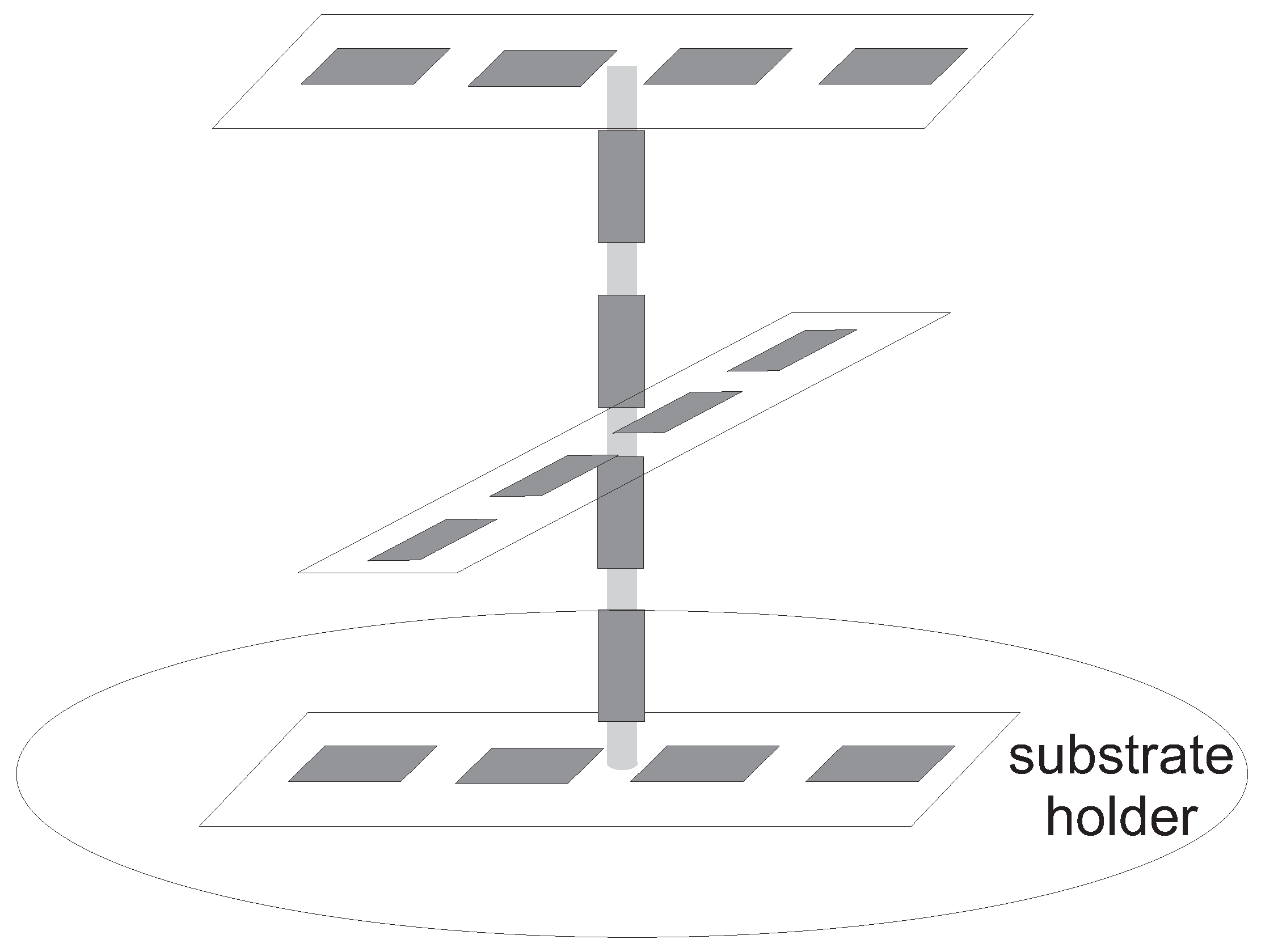
2.1. CVD Reactor
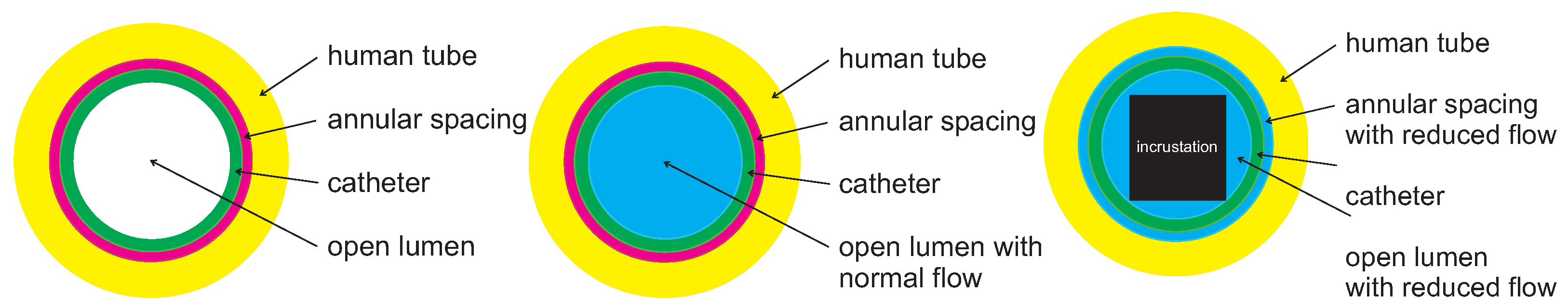
2.2. Ureteral Stent
2.3. Spatial Deposition Rate
2.4. Film Thickness
3. Results
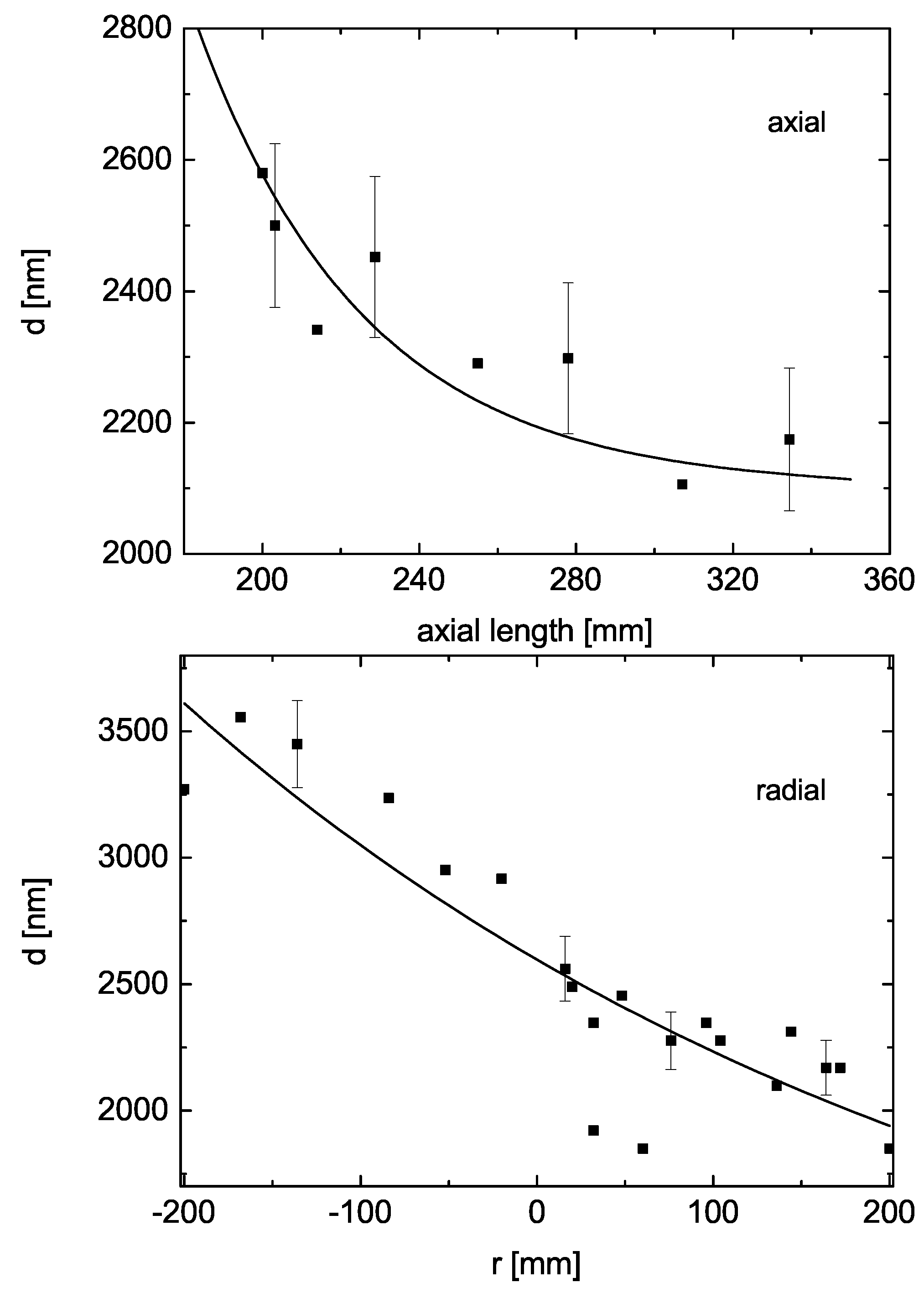
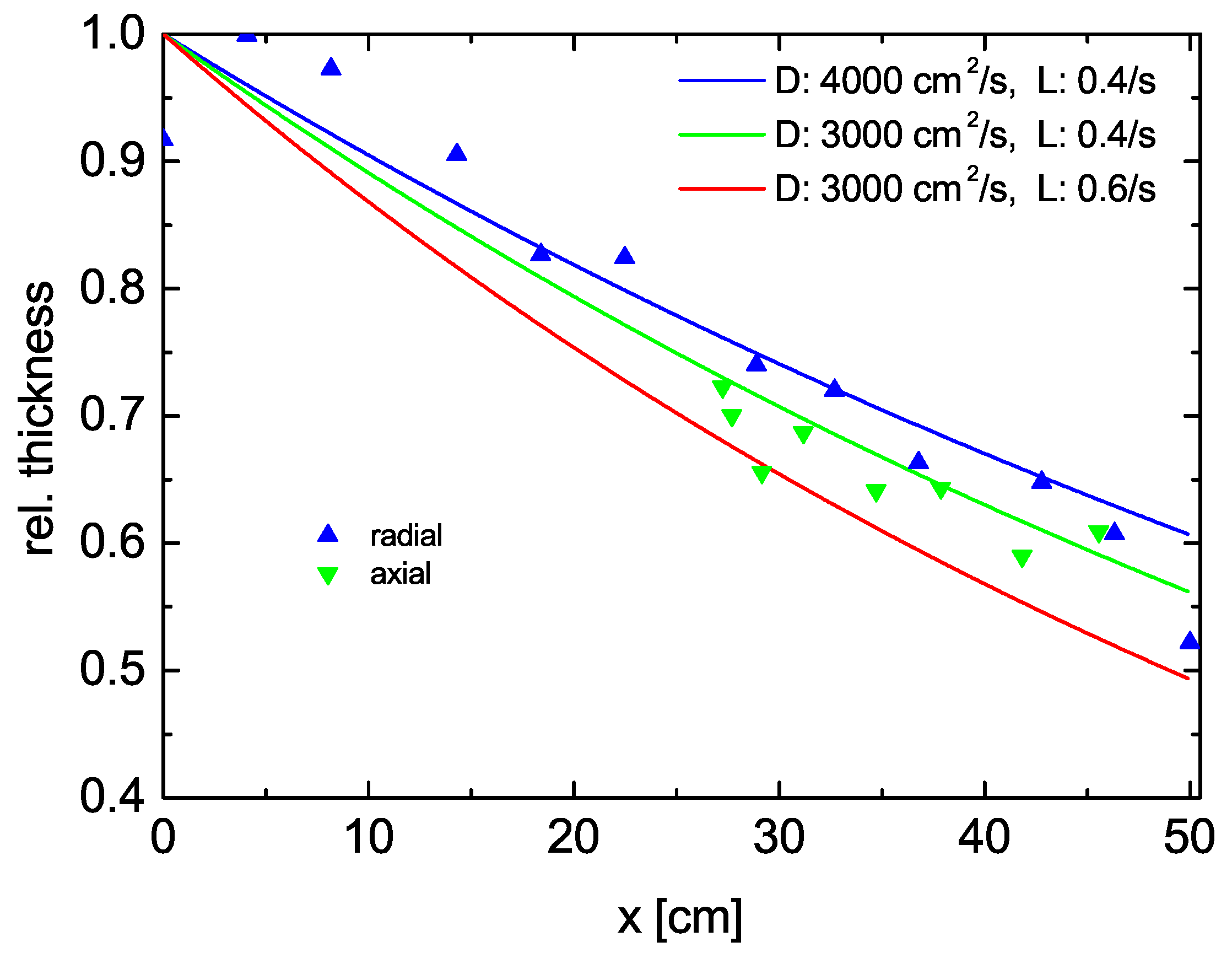
3.1. CVD in the Reactor
- The vertical distribution shows higher values at the top.
- The radial distribution shows an influence of the entrance gate at the left (at negative radii), Figure 7, which are numerically fitted with an erfc function.
- (losses dominate diffusion);
- (no losses).
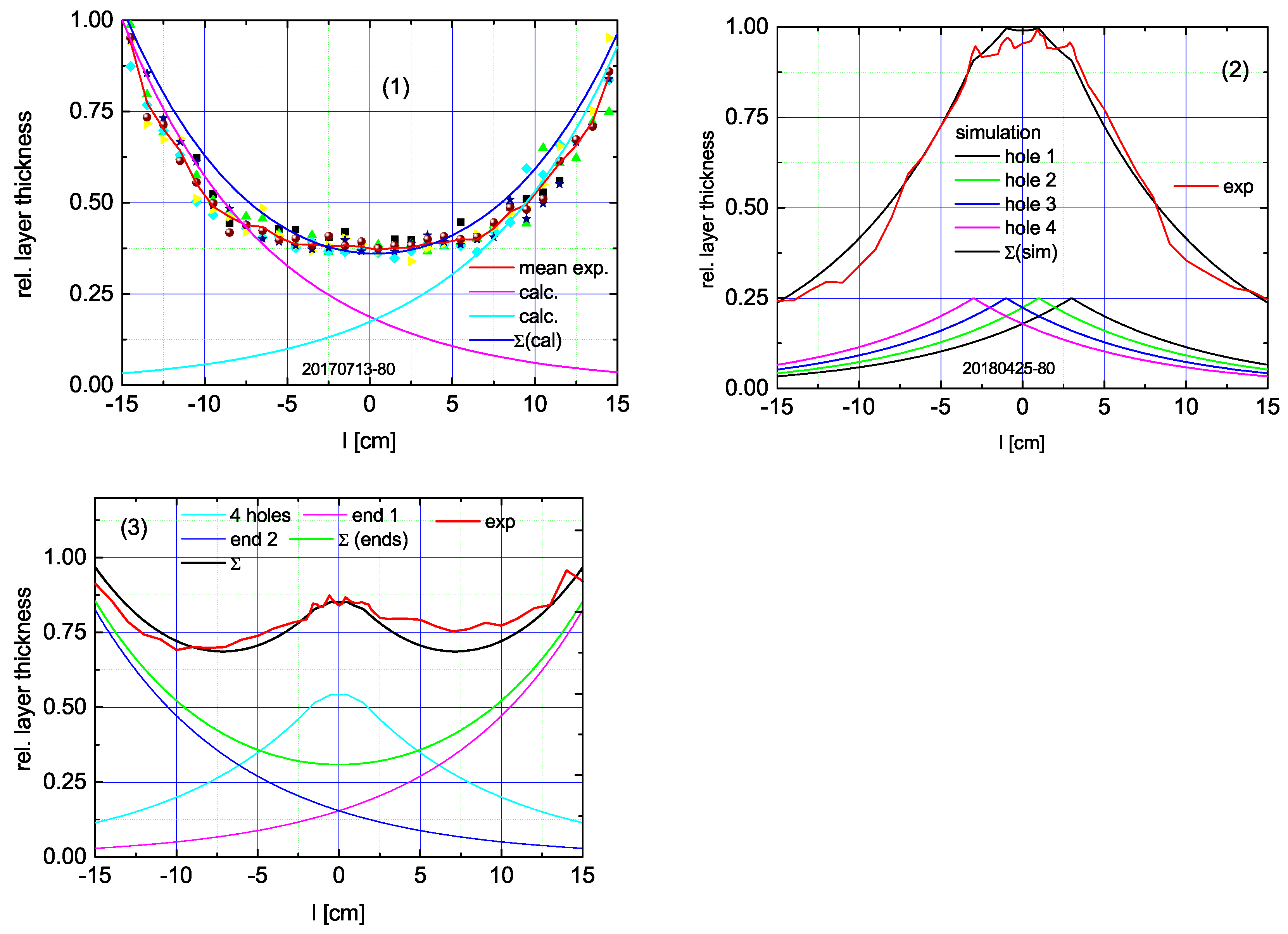
3.2. CVD in the Tube
- 1.
- Both ends open;
- 2.
- Both ends closed but 4 equidistant drainage openings in the middle;
- 3.
- Both ends open and 4 drainage openings.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devanathan, D.; Carr, R. Polymeric Conformal Coatings for Implantable Electronic Devices. IEEE Trans. Biomed. Eng. 1980, BME-27, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.A. (Ed.) Plastics in coatings and finishes. In Handbook of Plastics, Elastomers, and Composites, 4th ed.; McGraw Hill, Inc.: New York, NY, USA, 2002; Chapter 6. [Google Scholar]
- Licari, J.J. (Ed.) Coating Materials for Electronic Applications; Noyes Publications: Park City, NJ, USA, 2003. [Google Scholar]
- Kahn, R.H.; Burkel, W.E. Propagation of pseudo-intimal linings of vascular prostheses. In Vitro 1973, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A. Conformal Coatings for Pacemaker Applications; NBS Special Publication, U.S. Government Printing Office: Washington, DC, USA, 1979; Volume 400–450, pp. 109–113, Library of Congress Catalog Number: 78-600158.
- Franz, G.; Schamberger, F.; Heidari Zare, H.; Bröskamp, S.F.; Jocham, D. Bi-layer sandwich film for antibacterial catheters. Beilstein J. Nanotechnol. 2017, 8, 1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, J.-L. Nosocomial infections in adult intensive-care units. Lancet 2003, 361, 2068–2077. [Google Scholar] [CrossRef]
- Franz, G.; Schamberger, F.; Kutschera, A.; Seyedi, S.; Jocham, D. Strukturierte Beschichtung eines Substrats. German Patent Disclosure DE 10 2012 023 349.3, 29 November 2012. [Google Scholar]
- Franz, G.; Schamberger, F.; Jocham, D. Schichtstruktur zur Abgabe Mindestens eines Wirkstoffs. German Patent Disclosure DE 10 2012 023 343.3, 29 November 2012. [Google Scholar]
- Schwetlick, K. Organikum; Deutscher Verlag der Wissenschaften: Berlin, Germany, 1973; p. 722. [Google Scholar]
- Frischauf, P. Herstellung und Charakterisierung von Silberschichten auf Ureterschienen; Bachel. Th.: Munich, Germany, 2016. [Google Scholar]
- Heidari Zare, H.; Juhart, V.; Vass, A.; Franz, G.; Jocham, D. Efficacy of silver/hydrophilic poly(p-xylylene) on preventing bacterial growth and biofilm formation in urinary catheters. Biointerphases 2017, 12, 011001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interf. Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Gorham, W.F. A New, General Synthetic Method for the Preparation of Linear Poly-p-xylylenes. J. Polym. Sci. Part A-1 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Franz, G.; Heidari Zare, H.; Resch, K. Beschichtung der Innenwandung von Schlauchförmigen Substraten. German Patent Disclosure DE 10 2014 019 238 A1 2016.06.23, 23 June 2016. [Google Scholar]
- Bröskamp, S.; Redka, D.; Möhlmann, A.; Franz, G.; Jocham, D. Chemical vapor deposition of poly-p-xylylene in narrow tubes. AIP Adv. 2017, 7, 075005. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.-R.; Ganguli, S.; Karcz, J.; Gill, W.N.; Lu, T.-M. High deposition rate parylene films. J. Crystal Growth 1998, 183, 385. [Google Scholar] [CrossRef]
- Yasuda, H.K.; Yeh, Y.S.; Fusselman, S. A growth mechanism for the vacuum deposition of polymeric materials. Pure Appl. Chem. 1990, 62, 1689–1698. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. A Model for the Chemical Vapor Deposition of Poly(para-xylylene) (Parylene) Thin Films. Chem. Mater. 2002, 14, 1945. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.-M. Chemical Vapor Deposition Polymerization; Kluwer Academic Publishers: Boston, MA, USA, 2004; p. 45. [Google Scholar]
- Vaeth, K.M.; Jensen, K.F. Transition Metals for Selective Chemical Vapor Deposition of Parylene-Based Polymers. Chem. Mater. 2000, 12, 1305–1313. [Google Scholar] [CrossRef]
- Franz, G.; Schamberger, F. Evaporation and thermal cracking of dimeric parylenes. J. Vac. Sci. Technol. 2013, 31, 061602. [Google Scholar] [CrossRef]
- Franz, G.; Schamberger, F.; Voss, D. Druckgesteuerte Abscheiderate. German Patent Disclosure DE 2012 014 915.8, 29 July 2012. [Google Scholar]
- Li, J.-M.; Talu, O. Effect of structural heterogeneity on multicomponent adsorption: Benzene and p-xylene mixture on silicalite. In Fundamentals of Adsorption; Suzuki, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 373–380. ISBN 0080887724. [Google Scholar]
- Beach, W.F. A Model for the Vapor Deposition Polymerization of p-Xylylene. Macromolecules 1978, 11, 72–76. [Google Scholar] [CrossRef]
- Redka, D. priv. comm. Personal communication, 15 April 2021. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bröskamp, S.F.; Franz, G.; Jocham, D. Internal Coating of Ureteral Stents with Chemical Vapor Deposition of Parylene. Coatings 2021, 11, 739. https://doi.org/10.3390/coatings11060739
Bröskamp SF, Franz G, Jocham D. Internal Coating of Ureteral Stents with Chemical Vapor Deposition of Parylene. Coatings. 2021; 11(6):739. https://doi.org/10.3390/coatings11060739
Chicago/Turabian StyleBröskamp, Sara Felicitas, Gerhard Franz, and Dieter Jocham. 2021. "Internal Coating of Ureteral Stents with Chemical Vapor Deposition of Parylene" Coatings 11, no. 6: 739. https://doi.org/10.3390/coatings11060739






