Iridin Induces G2/M Phase Cell Cycle Arrest and Extrinsic Apoptotic Cell Death through PI3K/AKT Signaling Pathway in AGS Gastric Cancer Cells
Abstract
:1. Introduction
2. Results
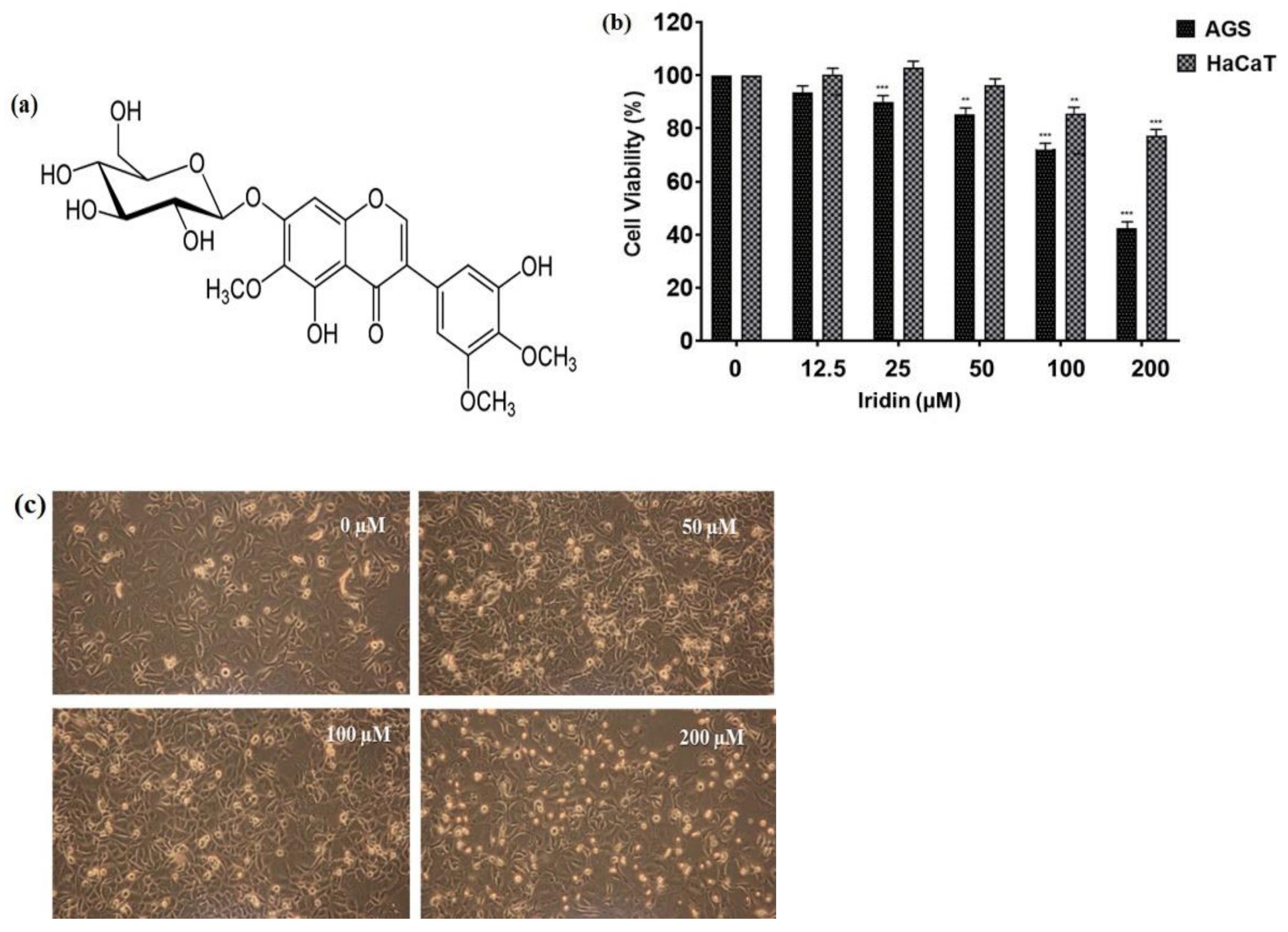
2.1. Iridin Inhibits Cell Proliferation and Triggers Cell Death in AGS Cells
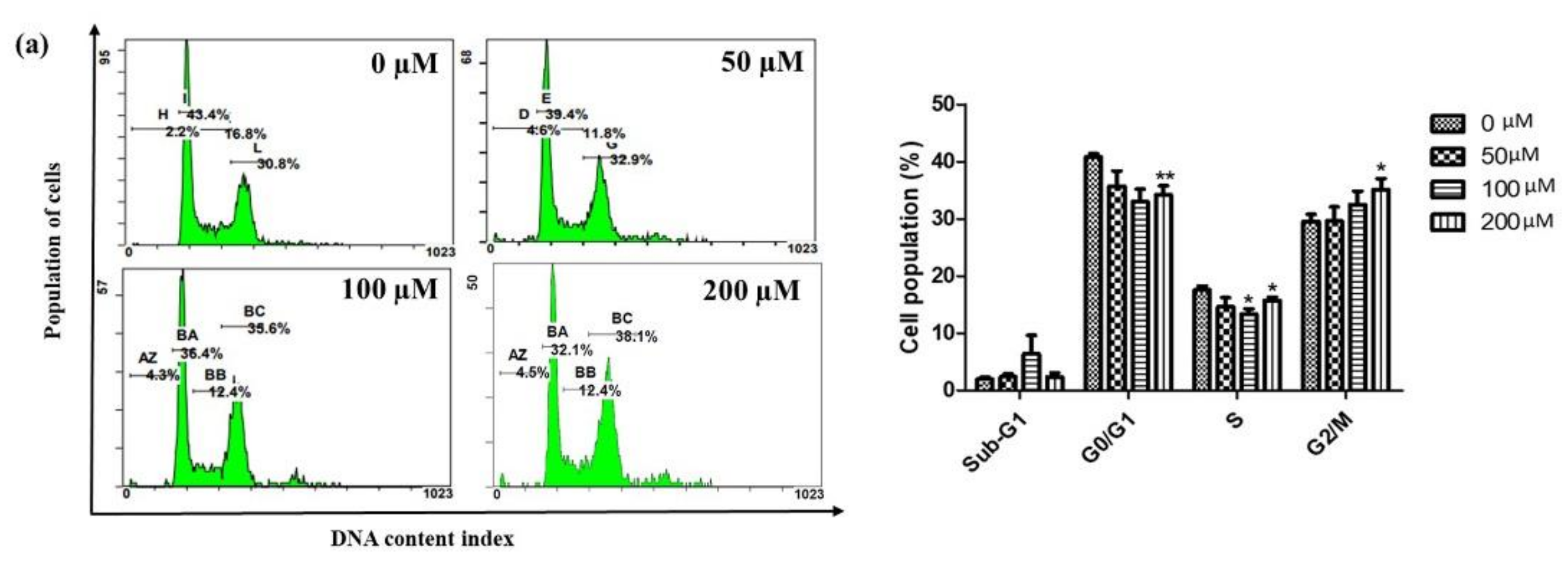
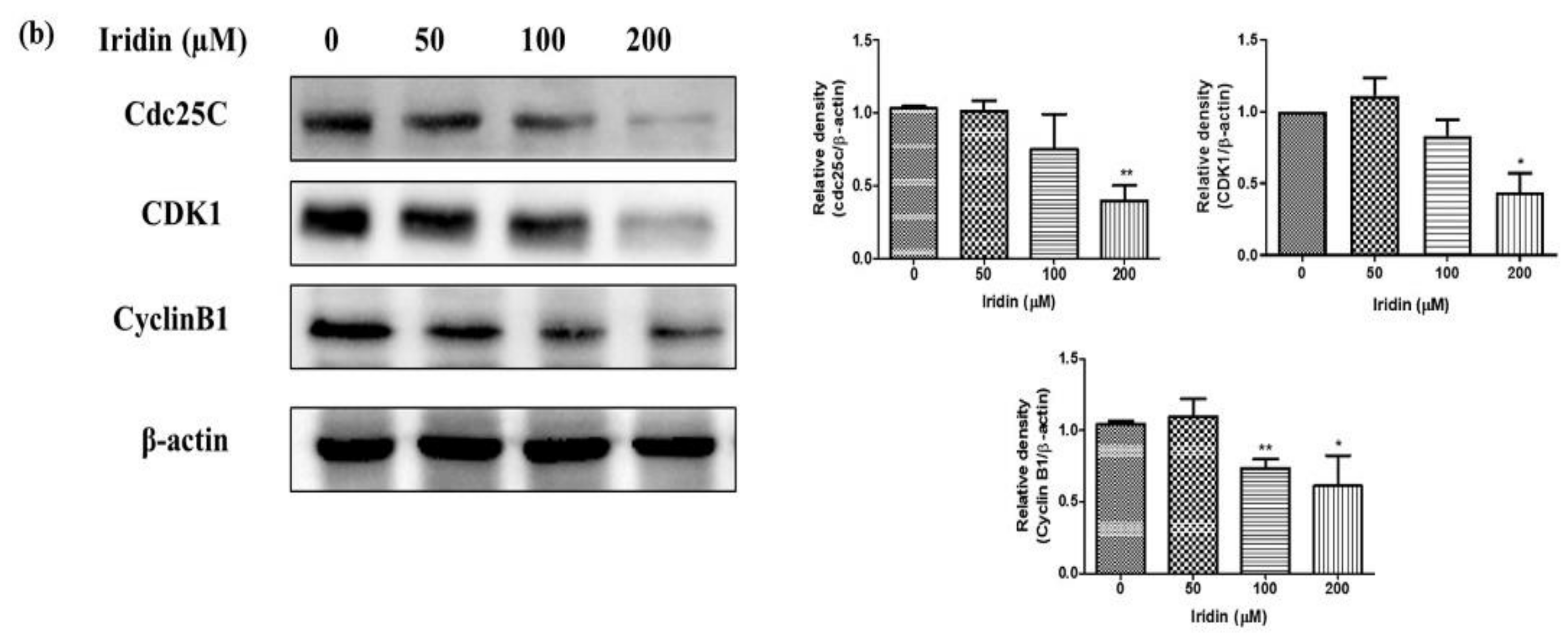
2.2. Iridin Induces G2/M Phase Cell Cycle Arrest by Modulating Cyclin B1, Cdc25C, and CDK1 Protein Expression in AGS Cells
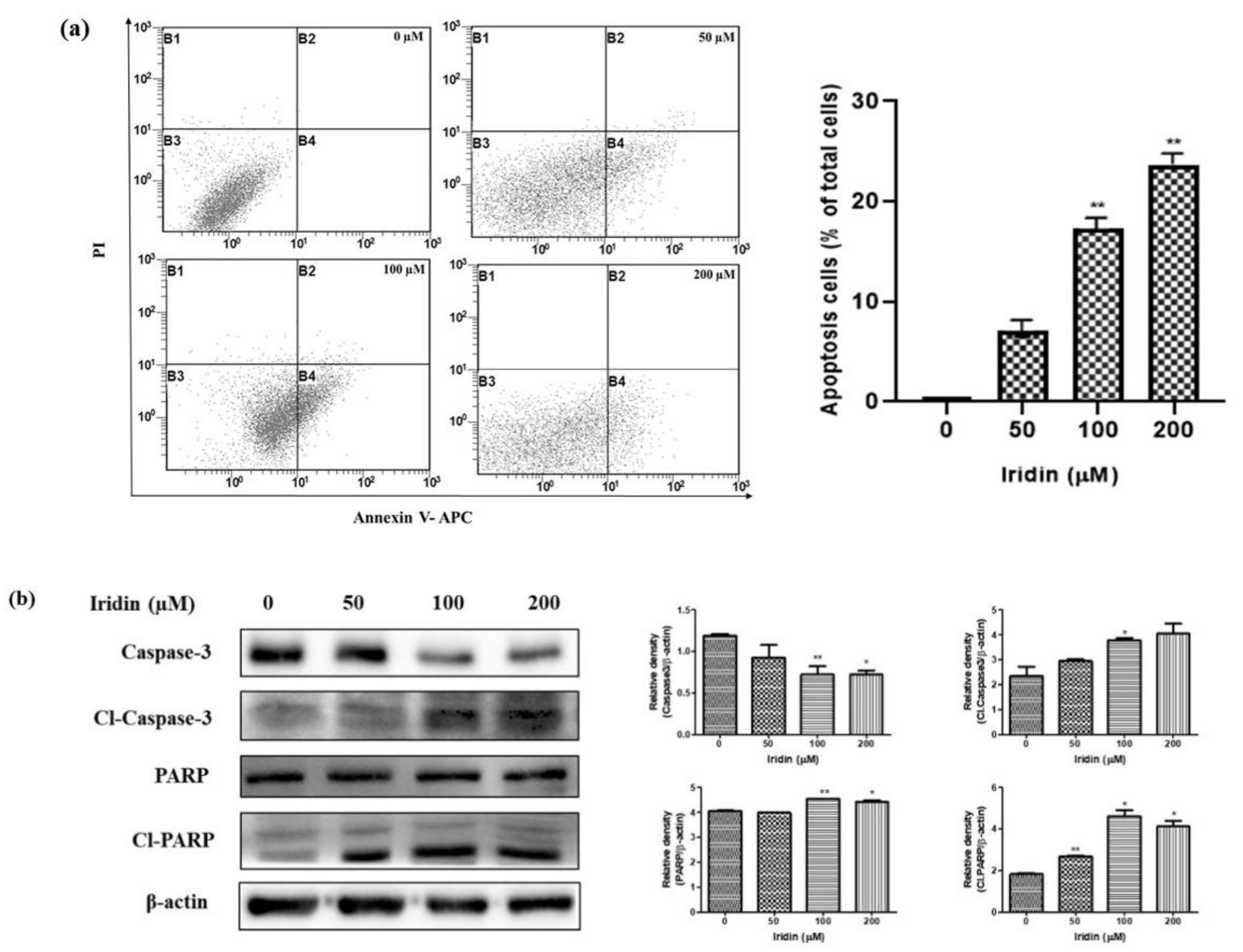
2.3. Iridin Induces Apoptosis in AGS Cells
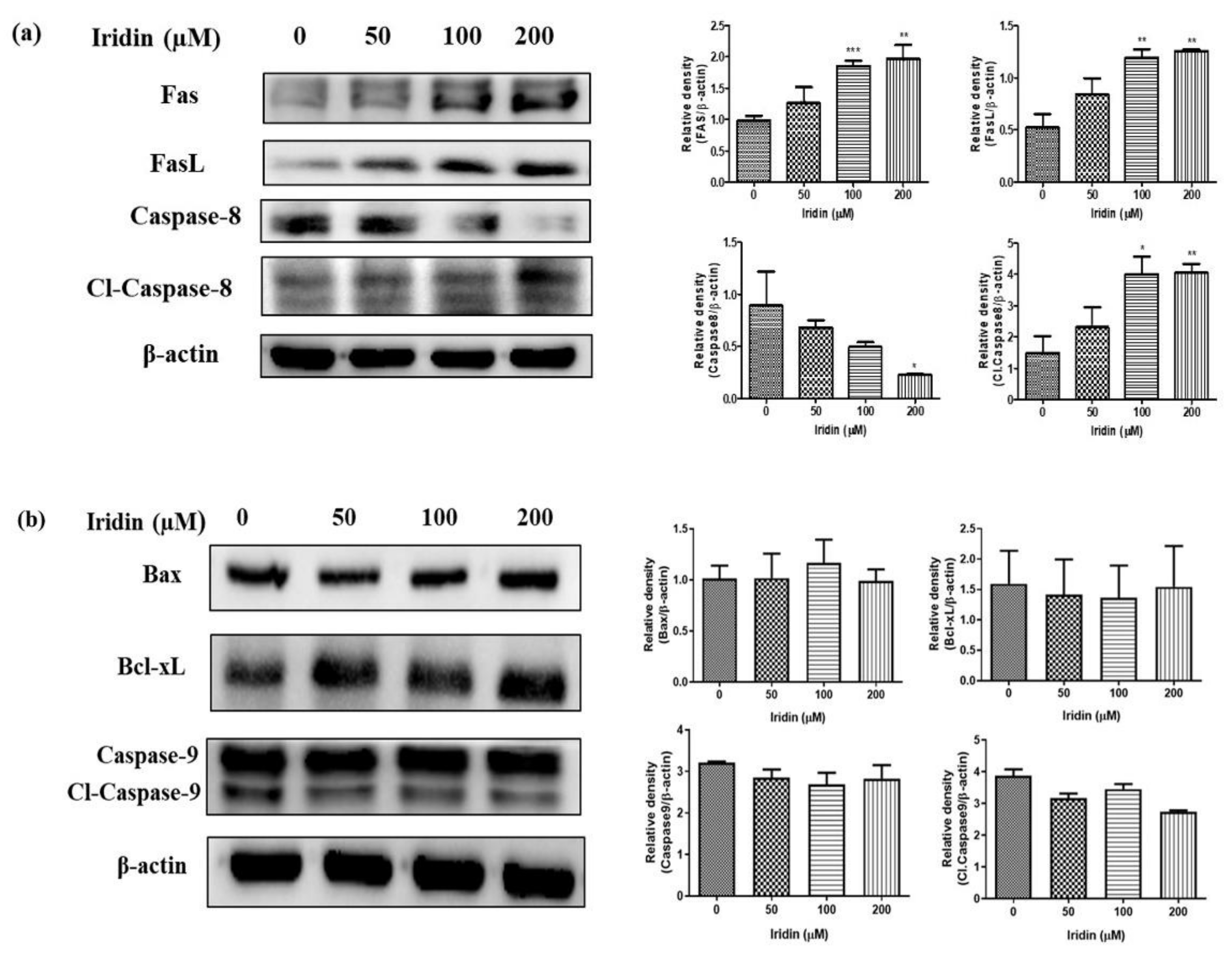
2.4. Iridin Induces Extrinsic Apoptotic Pathway
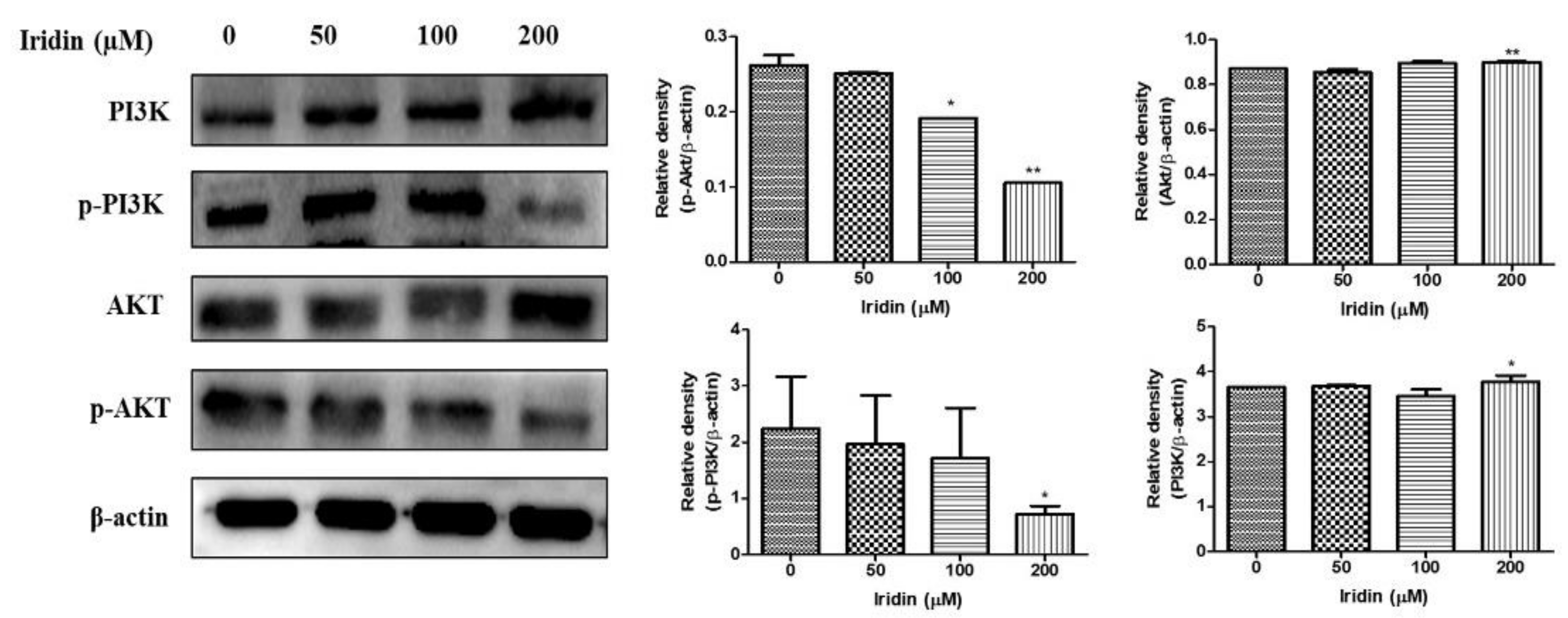
2.5. Effect of Iridin on PI3K/AKT Signaling Pathway in AGS Cells
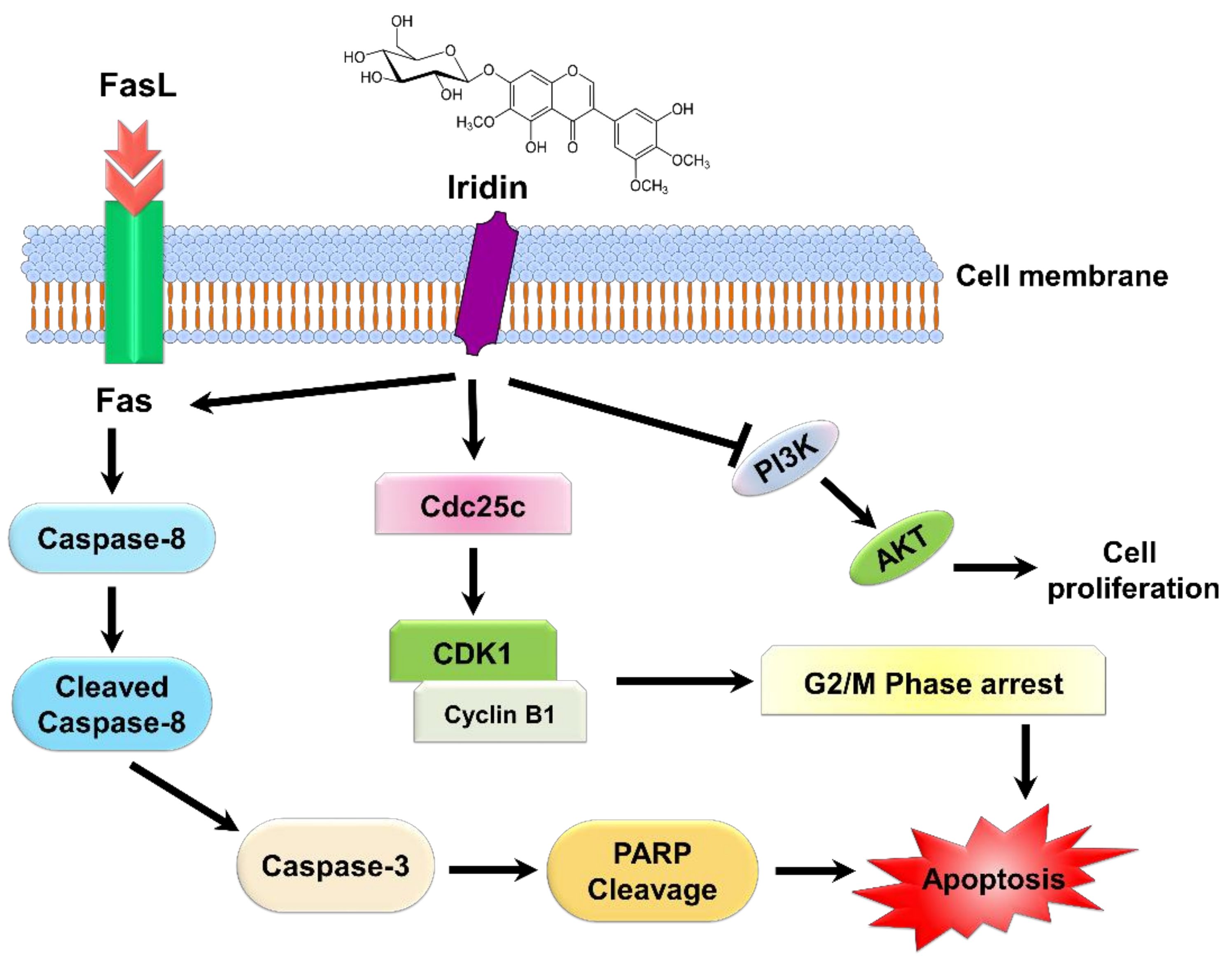
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Iridin Treatment
4.3. Cell Viability Assay
4.4. Analysis of Cell Cycle Distribution Using Flow Cytometry
4.5. Annexin V Propidium Iodide Apoptosis Detection
4.6. Western Blotting Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Yen, C.-W.; Xu, H.-W.; Deng, Y.-F.; Lin, Y.-J.; Hsu, W.-T.; Wu, C.-S.; Li, C. Upregulation of bone morphogenetic protein 1 is associated with poor prognosis of late-stage gastric Cancer patients. BMC Cancer 2018, 18, 508. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017, 39, 1010428317714626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre, J.; Garcia-Saenz, J.A.; Diaz-Rubio, E. Chemotherapy for gastric cancer. World J. Gastroenterol. 2006, 12, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [Green Version]
- Strong, V.E. Progress in gastric cancer. Updates Surg. 2018, 70, 157–159. [Google Scholar] [CrossRef] [Green Version]
- Poon, R.Y.C. Cell cycle control: A system of interlinking oscillators. Methods Mol. Biol. 2016, 1342, 3–19. [Google Scholar] [CrossRef]
- Clere, N.; Faure, S.; Martinez, M.C.; Andriantsitohaina, R. Anticancer properties of flavonoids: Roles in various stages of carcinogenesis. Cardiovasc. Hematol. Agents Med. Chem. 2011, 9, 62–77. [Google Scholar] [CrossRef] [Green Version]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Shendge, A.K.; Chaudhuri, D.; Mandal, N. The natural flavones, acacetin and apigenin, induce Cdk-Cyclin mediated G2/M phase arrest and trigger ROS-mediated apoptosis in glioblastoma cells. Mol. Biol. Rep. 2021, 48, 539–549. [Google Scholar] [CrossRef]
- Yang, G.; Yin, X.; Ma, D.; Su, Z. Anticancer activity of Phloretin against the human oral cancer cells is due to G0/G1 cell cycle arrest and ROS mediated cell death. J. BUON 2020, 25, 344–349. [Google Scholar]
- Kim, S.M.; Vetrivel, P.; Ha, S.E.; Kim, H.H.; Kim, J.-A.; Kim, G.S. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J. Nutr. Biochem. 2020, 83, 108427. [Google Scholar] [CrossRef]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Devel. Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Wei, X.; Qian, J.; Mo, X.; Kai, G.; An, F.; Lu, Y. 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone induced apoptosis and G1 cell cycle arrest through PI3K/AKT pathway in BEL-7402/5-FU cells. Food Chem. Toxicol. 2019, 131, 110533. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and smoking-related cancer risk: A meta-analysis. PLoS ONE 2013, 8, e75604. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Kim, S.H.; Kim, J.K.; Lee, S.; Jung, S.H.; Lim, S.S. Preparative isolation and purification of seven isoflavones from Belamcanda chinensis. Phytochem. Anal. 2011, 22, 468–473. [Google Scholar] [CrossRef]
- Morrissey, C.; Bektic, J.; Spengler, B.; Galvin, D.; Christoffel, V.; Klocker, H.; Fitzpatrick, J.M.; Watson, R.W.G. Phytoestrogens derived from Belamcanda chinensis have an antiproliferative effect on prostate cancer cells in vitro. J. Urol. 2004, 172, 2426–2433. [Google Scholar] [CrossRef]
- Sengupta, R.; Barone, A.; Marasa, J.; Taylor, S.; Jackson, E.; Warrington, N.M.; Rao, S.; Kim, A.H.; Leonard, J.R.; Piwnica-Worms, D.; et al. Novel chemical library screen identifies naturally occurring plant products that specifically disrupt glioblastoma-endothelial cell interactions. Oncotarget 2015, 6, 18282–18292. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Al-Ahdal, A.; Khedr, A.; Mohamed, G. Antioxidant α-amylase inhibitors flavonoids from Iris germanica rhizomes. Rev. Bras. Farmacogn. 2017, 27, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.-W.; Zeng, Z.-Q.; Shi, H.-L.; Liang, J.; Liu, Y.-M.; Tang, Y.-X.; Liao, X. Characterization of in vitro metabolites of irisflorentin 1by rat liver microsomes using high-performance liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2016, 30, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, S.; Jin, L.; Hu, D.; Wu, Z.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef]
- Saralamma, V.V.G.; Lee, H.J.; Hong, G.E.; Park, H.S.; Yumnam, S.; Raha, S.; Lee, W.S.; Kim, E.H.; Sung, N.J.; Lee, S.J.; et al. Korean Scutellaria baicalensis Georgi flavonoid extract induces mitochondrially mediated apoptosis in human gastric cancer AGS cells. Oncol. Lett. 2017, 14, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Park, H.S.; Kim, J.A.; Hong, G.E.; Nagappan, A.; Park, K.I.; Kim, G.S. Flavonoids identified from Korean Scutellaria baicalensis induce apoptosis by ROS generation and caspase activation on human fibrosarcoma cells. Am. J. Chin. Med. 2014, 42, 465–483. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Seol, D.-W. The role of mitochondria in apoptosis. BMB Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaudan, A.; Sharma, S.; Malek, S.N.A.; Dixit, A. Induction of apoptosis by pinostrobin in human cervical cancer cells: Possible mechanism of action. PLoS ONE 2018, 13, e0191523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, E.H.; Seong, M.K.; Ho, J.L.; Vetrivel, P.; Venkatarame, G.S.V.; Jeong, D.H.; Eun, H.K.; Sang, J.L.; Gon, S.K. Scutellarein induces fas-mediated extrinsic apoptosis and G2/M Cell cycle arrest in Hep3B hepatocellular carcinoma cells. Nutrients 2019, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Fleisher, T.A. Apoptosis. Ann. Allergy Asthma Immunol. 1997, 78, 245–249. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual role of Fas/FasL-Mediated signal in peripheral immune tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Saralamma, V.V.G.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Yumnam, S.; Raha, S.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H.; et al. Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of fas ligand. Int. J. Mol. Sci. 2015, 16, 22676–22691. [Google Scholar] [CrossRef] [Green Version]
- Rachakhom, W.; Khaw-On, P.; Pompimon, W.; Banjerdpongchai, R. Dihydrochalcone derivative induces breast cancer cell apoptosis via intrinsic, extrinsic, and ER stress pathways but abolishes EGFR/MAPK pathway. Biomed. Res. Int. 2019, 2019, 7298539. [Google Scholar] [CrossRef]
- Elango, R.; Athinarayanan, J.; Subbarayan, V.P.; Lei, D.K.Y.; Alshatwi, A.A. Hesperetin induces an apoptosis-triggered extrinsic pathway and a p53- independent pathway in human lung cancer H522 cells. J. Asian Nat. Prod. Res. 2018, 20, 559–569. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef]
- Jia, X.; Wen, Z.; Sun, Q.; Zhao, X.; Yang, H.; Shi, X.; Xin, T. Apatinib suppresses the proliferation and apoptosis of gastric cancer cells via the PI3K/Akt signaling pathway. J. BUON 2019, 24, 1985–1991. [Google Scholar]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weng, Q.; Chen, J.; Han, J. Morusin inhibits human osteosarcoma via the PI3K-AKT signaling pathway. Curr. Pharm. Biotechnol. 2020, 21, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Bak, Y.; Kim, H.; Kang, J.W.; Lee, D.H.; Kim, M.S.; Park, Y.S.; Kim, J.H.; Jung, K.Y.; Lim, Y.; Hong, J. A synthetic naringenin derivative, 5-hydroxy-7,4′-diacetyloxyflavanone-N-phenyl hydrazone (N101-43), induces apoptosis through up-regulation of Fas/FasL expression and inhibition of PI3K/Akt signaling pathways in non-small-cell lung cancer cells. J. Agric. Food Chem. 2011, 59, 10286–10297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, P.-B.; Vetrivel, P.; Ha, S.-E.; Kim, H.-H.; Heo, J.-D.; Won, C.-K.; Kim, S.-M.; Kim, G.-S. Iridin Induces G2/M Phase Cell Cycle Arrest and Extrinsic Apoptotic Cell Death through PI3K/AKT Signaling Pathway in AGS Gastric Cancer Cells. Molecules 2021, 26, 2802. https://doi.org/10.3390/molecules26092802
Bhosale P-B, Vetrivel P, Ha S-E, Kim H-H, Heo J-D, Won C-K, Kim S-M, Kim G-S. Iridin Induces G2/M Phase Cell Cycle Arrest and Extrinsic Apoptotic Cell Death through PI3K/AKT Signaling Pathway in AGS Gastric Cancer Cells. Molecules. 2021; 26(9):2802. https://doi.org/10.3390/molecules26092802
Chicago/Turabian StyleBhosale, Pritam-Bhagwan, Preethi Vetrivel, Sang-Eun Ha, Hun-Hwan Kim, Jeong-Doo Heo, Chung-Kil Won, Seong-Min Kim, and Gon-Sup Kim. 2021. "Iridin Induces G2/M Phase Cell Cycle Arrest and Extrinsic Apoptotic Cell Death through PI3K/AKT Signaling Pathway in AGS Gastric Cancer Cells" Molecules 26, no. 9: 2802. https://doi.org/10.3390/molecules26092802





