Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
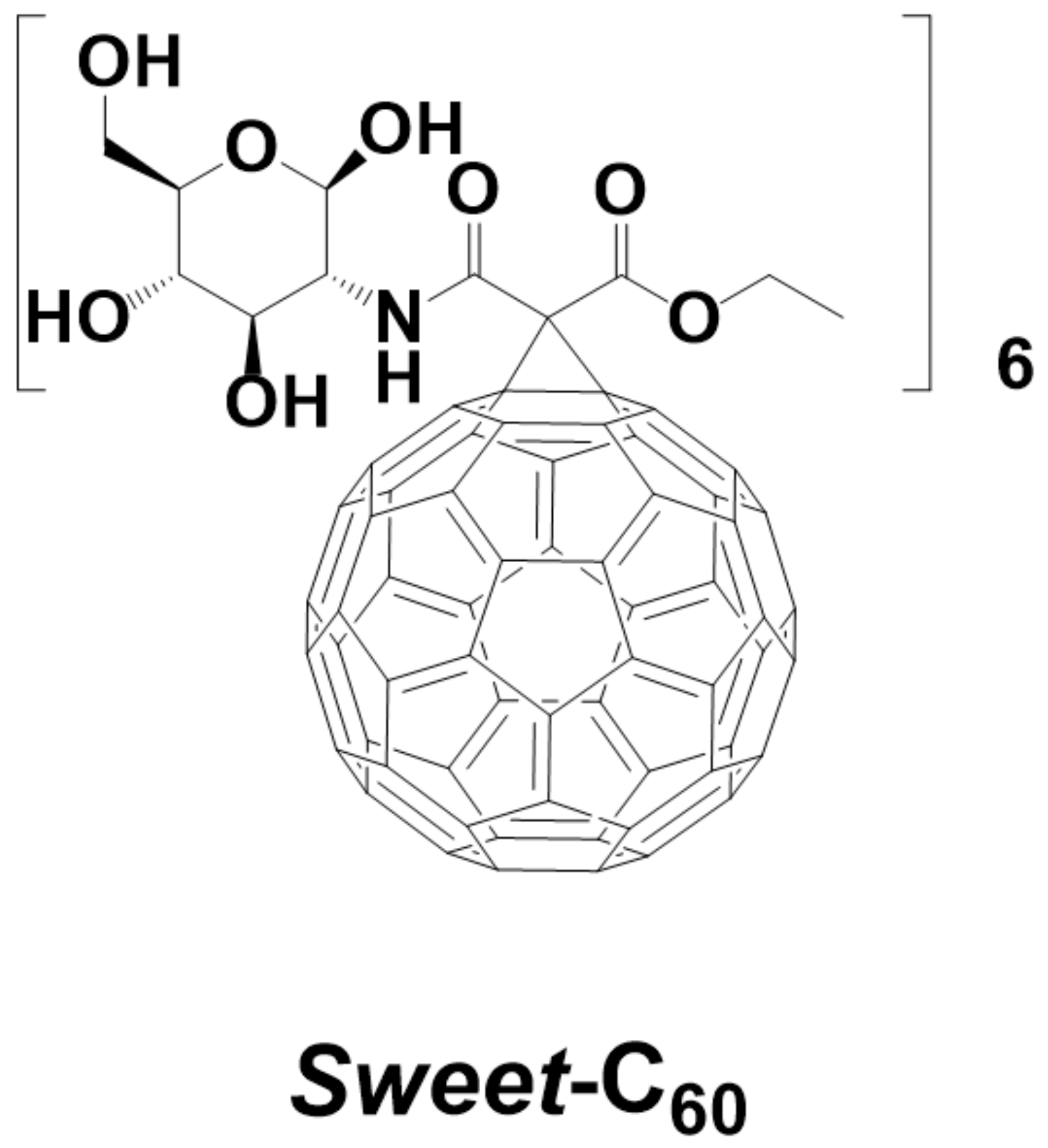
2.2. Glycofullerene Synthesis and Application
2.3. Cell Counting Using a Hemocytometer
2.4. The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assays
2.5. Wound-Healing Assay
2.6. Cell Migration
2.7. Western Blot
2.7.1. Procedure for the GLUT-1 Protein
2.7.2. Procedure for the HIF-1α, HO-1 and MMP-2 Proteins
2.8. Glycolysis Assay (Extracellular Acidification)
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Changes in the Rate of Proliferation
3.2. The Migration Rate and Wound Healing
3.3. Expression of the Glucose Transporter Protein (GLUT-1)
3.4. Expression of the Proteins Related to Cancer Cell Migration
3.5. Glycolysis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acquah, S.F.A.; Penkova, A.V.; Markelov, D.A.; Semisalova, A.S.; Leonhardt, B.E.; Magi, J.M. Review—The Beautiful Molecule: 30 Years of C60and Its Derivatives. ECS J. Solid State Sci. Technol. 2017, 6, M3155–M3162. [Google Scholar] [CrossRef]
- D’Amora, M.; Giordani, S. Carbon Nanomaterials for Nanomedicine. In Smart Nanoparticles for Biomedicine; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 103–113. [Google Scholar]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in combating cancer: Therapeutic applications and developments. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A. Water-soluble fullerenes using solubilizing agents, and their applications. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 49–65. [Google Scholar] [CrossRef]
- Hotze, E.M.; Labille, J.; Alvarez, P.; Wiesner, M.R. Mechanisms of photochemistry and reactive oxygen production by full-erene suspensions in water. Environ. Sci. Technol. 2008, 42, 4175–4180. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenesin vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Wenz, G. Molecular solubilization of fullerene C60 in water by γ-cyclodextrin thioethers. Beilstein J. Org. Chem. 2012, 8, 1644–1651. [Google Scholar] [CrossRef] [Green Version]
- Serda, M.; Ware, M.J.; Newton, J.M.; Sachdeva, S.; Krzykawska-Serda, M.; Nguyen, L.; Law, J.; O Anderson, A.; A Curley, S.; Wilson, L.J.; et al. Development of photoactive Sweet-C60 for pancreatic cancer stellate cell therapy. Nanomedicine 2018, 13, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, P.; Gawecki, R.; Szewczyk, G.; Balin, K.; Dulski, M.; Sajewicz, M.; Mrozek-Wilczkiewicz, A.; Musioł, R.; Polanski, J.; Serda, M. A [60]fullerene nanoconjugate with gemcitabine: Synthesis, biophysical properties and biological evaluation for treating pancreatic cancer. Cancer Nanotechnol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Serda, M.; Szewczyk, G.; Krzysztyńska-Kuleta, O.; Korzuch, J.; Dulski, M.; Musioł, R.; Sarna, T. Developing [60] Fullerene Nanomaterials for Better Photodynamic Treatment of Non-Melanoma Skin Cancers. ACS Biomater. Sci. Eng. 2020, 6, 5930–5940. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, P.; Chen, Y.; Zhao, Y.-L.; Ding, F.; Yu, A. Spectrophotometric Study of Fluorescence Sensing and Selective Binding of Biochemical Substrates by 2,2‘-Bridged Bis(β-cyclodextrin) and Its Water-Soluble Fullerene Conjugate. J. Phys. Chem. B 2005, 109, 23739–23744. [Google Scholar] [CrossRef]
- Lapin, N.A.; Krzykawska-Serda, M.; Dilliard, S.; Mackeyev, Y.; Serda, M.; Wilson, L.J.; Curley, S.A.; Corr, S.J. The effects of non-invasive radiofrequency electric field hyperthermia on biotransport and biodistribution of fluorescent [60] fullerene derivative in a murine orthotopic model of breast adenocarcinoma. J. Control. Release 2017, 260, 92–99. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as photosensitizers in photodynamic therapy: Pros and cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef]
- Minami, K.; Okamoto, K.; Harano, K.; Noiri, E.; Nakamura, E. Hierarchical Assembly of siRNA with Tetraamino Fullerene in Physiological Conditions for Efficient Internalization into Cells and Knockdown. ACS Appl. Mater. Interfaces 2018, 10, 19347–19354. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Zhou, Y.; Liu, S.; Chen, D.; Ge, J.; Deng, R.; Li, X.; Yu, T.; Xu, H.; Sun, D.; et al. Photo-triggered gadofullerene: Enhanced cancer therapy by combining tumor vascular disruption and stimulation of anti-tumor immune responses. Biomaterials 2019, 213, 119218. [Google Scholar] [CrossRef]
- Serda, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Wojtyniak, M.; Musioł, R.; Curley, S.A. Glycofullerenes as non-receptor tyrosine kinase inhibitors—towards better nanotherapeutics for pancreatic cancer treatment. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yang, X.; Ebrahimi, A.; Li, J. Fullerene–biomolecule conjugates and their biomedicinal applications. Int. J. Nanomed. 2013, 9, 77–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ye, C.; Chen, C.; Xiong, H.; Xie, B.; Zhou, J.; Chen, Y.; Zheng, S.; Wang, L. Glucose transporter GLUT1 expression and clinical outcome in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 16875–16886. [Google Scholar] [CrossRef] [Green Version]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Neesse, A.; Krug, S.; Gress, T.M.; Tuveson, D.A.; Michl, P. Emerging concepts in pancreatic cancer medicine: Targeting the tumor stroma. OncoTargets Ther. 2013, 7, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Pellegata, N.S.; Sessa, F.; Renault, B.; Bonato, M.; E Leone, B.; Solcia, E.; Ranzani, G.N. K-ras and p53 gene mutations in pancreatic cancer: Ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994, 54, 1556–1560. [Google Scholar]
- Bloomston, M.; Frankel, W.L.; Petrocca, F.; Volinia, S.; Alder, H.; Hagan, J.P.; Liu, C.-G.; Bhatt, D.; Taccioli, C.; Croce, C.M. MicroRNA Expression Patterns to Differentiate Pancreatic Adenocarcinoma From Normal Pancreas and Chronic Pancreatitis. JAMA 2007, 297, 1901–1908. [Google Scholar] [CrossRef] [Green Version]
- Polireddy, K.; Chen, Q. Cancer of the Pancreas: Molecular Pathways and Current Advancement in Treatment. J. Cancer 2016, 7, 1497–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009, 64, 489–500. [Google Scholar]
- Koong, A.; Mehta, V.K.; Le, Q.T.; Fisher, G.A.; Terris, D.J.; Brown, J.; Bastidas, A.J.; Vierra, M. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. 2000, 48, 919–922. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2020, 587, 358–366. [Google Scholar] [CrossRef]
- Duffy, J.P.; Eibl, G.; A Reber, H.; Hines, O.J. Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol. Cancer 2003, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, X.; Hou, Z.; Wang, N.; Jiang, Y.; Luan, Y. Engineering a photosensitizer nanoplatform for amplified photo-dynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horiz. 2021, 6, 120–131. [Google Scholar] [CrossRef]
- Koay, E.J.; Baio, F.E.; Ondari, A.; Truty, M.J.; Cristini, V.; Thomas, R.M.; Chen, R.; Chatterjee, D.; Kang, Y.; Zhang, J.; et al. Intra-tumoral heterogeneity of gemcitabine delivery and mass transport in human pancreatic cancer. Phys. Biol. 2014, 11, 065002. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, S.; Banerjee, S.; Chellappan, S.; Simon, G.R. Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett. 2007, 257, 244–251. [Google Scholar] [CrossRef]
- Ito, H.; Duxbury, M.; Zinner, M.J.; Ashley, S.W.; Whang, E.E. Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery 2004, 136, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Lapin, N.A.; Vergara, L.A.; Mackeyev, Y.; Newton, J.M.; Dilliard, S.A.; Wilson, L.J.; Curley, S.A.; Serda, R.E. Biotransport kinetics and intratumoral biodistribution of malonodiserinolamide-derivatized [60] fullerene in a murine model of breast adenocarcinoma. Int. J. Nanomed. 2017, 12, 8289–8307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoof, M.; Mackeyev, Y.; Cheney, M.A.; Wilson, L.J.; Curley, S.A. Internalization of C60 fullerenes into cancer cells with accumulation in the nucleus via the nuclear pore complex. Biomaterials 2012, 33, 2952–2960. [Google Scholar] [CrossRef] [Green Version]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Lieber, M.; Mazzetta, J.; Nelson-Rees, W.; Kaplan, M.; Todaro, G. Establishment of a continuous tumor-cell line (PANC-1) from a human carcinoma of the exocrine pancreas. Int. J. Cancer 1975, 15, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.L.; González-Hernández, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Marelli-Berg, F.M.; Fu, H.; Mauro, C. Molecular mechanisms of metabolic reprogramming in proliferating cells: Implica-tions for T-cell-mediated immunity. Immunology 2012, 136, 363–369. [Google Scholar] [CrossRef]
- Masur, K.; Vetter, C.; Hinz, A.; Tomas, N.; Henrich, H.; Niggemann, B.; Zänker, K. Diabetogenic glucose and insulin con-centrations modulate transcriptom and protein levels involved in tumour cell migration, adhesion and proliferation. Br. J. Cancer 2011, 104, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Lucafo, M.; Pelillo, C.; Carini, M.; Da Ros, T.; Prato, M.; Sava, G. A cationic [60] fullerene derivative reduces invasion and migration of HT-29 CRC cells in vitro at dose free of significant effects on cell survival. Nano Micro Lett. 2014, 6, 163–168. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, L.; Kang, S.-G.; Lu, Y.; Yang, Z.; Huynh, T.; Chen, C.; Zhou, R.; Guo, M.; Zhao, Y. Gd–metallofullerenol na-nomaterial suppresses pancreatic cancer metastasis by inhibiting the interaction of histone deacetylase 1 and metasta-sis-associated protein 1. ACS Nano 2015, 9, 6826–6836. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Voelker, H.-U.; Kapp, M.; Krockenberger, M.; Dietl, J.; Kämmerer, U. Glycolytic phenotype in breast cancer: Activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J. Cancer Res. Clin. Oncol. 2009, 136, 219–225. [Google Scholar] [CrossRef]
- Tekade, R.K.; Sun, X. The Warburg effect and glucose-derived cancer theranostics. Drug Discov. Today 2017, 22, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Park, H.-J.; Jeong, H.K.; Kim, M.-J.; Kim, M.; Bae, O.-N.; Baek, S.-H. Autophagy sustains the survival of human pancreatic cancer PANC-1 cells under extreme nutrient deprivation conditions. Biochem. Biophys. Res. Commun. 2015, 463, 205–210. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barańska, E.; Wiecheć-Cudak, O.; Rak, M.; Bienia, A.; Mrozek-Wilczkiewicz, A.; Krzykawska-Serda, M.; Serda, M. Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model. Nanomaterials 2021, 11, 513. https://doi.org/10.3390/nano11020513
Barańska E, Wiecheć-Cudak O, Rak M, Bienia A, Mrozek-Wilczkiewicz A, Krzykawska-Serda M, Serda M. Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model. Nanomaterials. 2021; 11(2):513. https://doi.org/10.3390/nano11020513
Chicago/Turabian StyleBarańska, Edyta, Olga Wiecheć-Cudak, Monika Rak, Aleksandra Bienia, Anna Mrozek-Wilczkiewicz, Martyna Krzykawska-Serda, and Maciej Serda. 2021. "Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model" Nanomaterials 11, no. 2: 513. https://doi.org/10.3390/nano11020513






