Fibril-Forming Organelles in Mesangial Cells in Renal Biopsies from Patients with Light-Chain-Associated Amyloidosis
Abstract
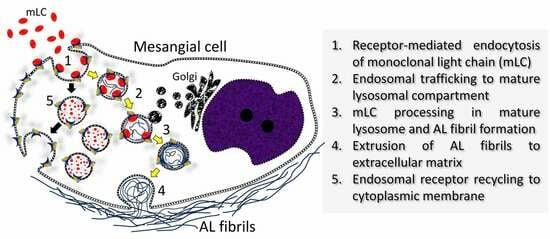
:1. Introduction
2. Material and Methods
2.1. Clinical Information
2.2. Light Microscopy
2.3. Immunofluorescence
2.4. Electron Microscopy
2.5. Ultrastructural Immunolabeling
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, G.A.; Teng, J.; Turbat-Herrera, E.A.; Zeng, C.; Del Pozo-Yauner, L. Understanding Mesangial Pathobiology in AL-Amyloidosis and Monoclonal Ig Light Chain Deposition Disease. Kidney Int. Rep. 2020, 5, 1870–1893. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.A. Renal amyloidosis: Pathogenesis. Ultrastruct. Pathol. 2021, 45, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sirac, C.; Herrera, G.A.; Sanders, P.W.; Batuman, V.; Bender, S.; Ayala, M.V.; Javaugue, V.; Teng, J.; Turbat-Herrera, E.A.; Cogné, M.; et al. Animal models of monoclonal immunoglobulin-related renal diseases. Nat. Rev. Nephrol. 2018, 14, 246–264. [Google Scholar] [CrossRef]
- Martinez-Rivas, G.; Bender, S.; Sirac, C. Understanding AL amyloidosis with a little help from in vivo models. Front. Immunol. 2022, 13, 1008449. [Google Scholar] [CrossRef]
- Picken, M.M. The pathology of amyloidosis in classification: A review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.A. Plasticity of mesangial cells: A basis for understanding pathological alterations. Ultrastruct Pathol. 2006, 30, 471–479. [Google Scholar] [CrossRef]
- Shirahama, T.; Cohen, A.S. An analysis of the close relationship of lysosomes to early deposits of amyloid. Ultrastructural evidence in experimental mouse amyloidosis. Am. J. Pathol. 1973, 73, 97–114. [Google Scholar]
- Shirahama, T.; Cohen, A.S. Intralysosomal formation of amyloid fibrils. Am. J. Pathol. 1975, 81, 101–116. [Google Scholar]
- Keeling, J.; Teng, J.; Herrera, G.A. AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab. Investig. 2004, 84, 1322–1338. [Google Scholar] [CrossRef]
- Del Pozo-Yauner, L.; Turbat-Herrera, E.A.; Pérez-Carre6n, J.; Herrera, G.A. From the Light Chain Sequence to the Tissue Microenvironment: Contribution of the Mesangial Cells to Glomerular Amyloidosis. Hemato 2022, 3, 232–267. [Google Scholar] [CrossRef]
- Herrera, G.A.; Del Pozo-Yauner, L.; Teng, J.; Zeng, C.; Shen, X.; Moriyama, T.; Ramirez Alcantara, V.; Liu, B.; Turbat-Herrera, E.A. Glomerulopathic light chain-mesangial cell interactions: Sortilin-related receptor (SORLI) and signaling. Kidney Int. Rep. 2021, 6, 1379–1396. [Google Scholar] [CrossRef]
- Isaac, J.; Kerby, J.D.; Russell, W.; Dempsey, S.C.; Sanders, P.W.; Herrera, G.A. In-vitro modulation of AL-amyloid formation by human mesangial cells exposed to amyloidogenic light chains. Amyloid Int. J. Exp. Clin. Investig. 1998, 5, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Sirac, C.; Terryn, S.; Javaugue, V.; Prange, J.A.; Bender, S.; Bonaud, A.; Cogné, M.; Aucouturier, P.; Ronco, P.; et al. Impaired lysosomal function underlies monoclonal light chain-associated Renal Fanconi Syndrome. J. Am. Soc. Nephrol. 2016, 27, 2049–2061. [Google Scholar] [CrossRef]
- Mai, H.L.; Sheikh-Hamad, D.; Herrera, G.A.; Gu, X.; Truong, L.D. Immunoglobulin heavy chain can be amyloidogenic: Morphologic characterization including immunoelectron microscopy. Am. J. Surg. Pathol. 2003, 27, 541–545. [Google Scholar] [CrossRef]
- Herrera, G.A.; Teng, J.; Zeng, C.; Pozo-Yauner, L.D.; Liu, B.; Turbat-Herrera, E.A. AL(light chain)-amyloidogenesis by mesangial cells involves active participation of lysosomes: An ultrastructural study. Heliyon 2023, 9, e15190. [Google Scholar] [CrossRef] [PubMed]
- von Gise, H.; Christ, H.; Bohle, A. Early glomerular lesions in amyloidosis. Electronmicroscopic findings. Virchows Arch. A Pathol. Anat. Histopathol. 1981, 390, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Linke, R.P.; Zucker-Franklin, D.; Franklin, E.D. Morphologic, chemical, and immunologic studies of amyloid-like fibrils formed from Bence Jones Proteins by proteolysis. J. Immunol. 1973, 111, 10–23. [Google Scholar] [CrossRef]
- Epstein, W.V.; Tan, M.; Wood, I.S. Formation of “amyloid” fibrils in vitro by action of human kidney lysosomal enzymes on Bence Jones proteins. J. Lab. Clin. Med. 1974, 84, 107–110. [Google Scholar]
- Teng, J.; Turbat-Herrera, E.A.; Herrera, G.A. Extrusion of amyloid fibrils to the extracellular space in experimental mesangial AL-amyloidosis: Transmission and scanning electron microscopy studies and correlation with renal biopsy observations. Ultrastruct Pathol. 2014, 38, 104–115. [Google Scholar] [CrossRef]
- Keeling, J.; Herrera, G.A. Matrix metalloproteinases and mesangial remodeling in light chain-related glomerular damage. Kidney Int. 2005, 68, 1590–1603. [Google Scholar] [CrossRef]
- De Duve, C.; Watt-iaux, R. Functions of lysosomes. Annu. Rev. Physiol. 1966, 28, 435–492. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Dennemärker, J.; Reinheckel, T. Specific functions of lysosomal proteases in endocytic and autophagic pathways. Biochim. Biophys. Acta 2012, 1824, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Masuyama, N.; Kuronita, T.; Fujita, H.; Himeno, M.; Tanaka, Y. Analysis of post-lysosomal compartments. Biochem. Biophys. Res. Commun. 2004, 314, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Frangione, B.; Franklin, E.C. Bence Jones proteins and light chains of immunoglobulins. Preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda). J. Clin. Investig. 1982, 70, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Comenzo, R.L.; Zhang, Y.; Martinez, C.; Osman, K.; Herrera, G.A. The tropism of organ involvement in primary systemic amyloidosis: Contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood 2001, 98, 714–720. [Google Scholar] [CrossRef]
- Teng, J.; Turbat-Herrera, E.A.; Herrera, G.A. An animal model of glomerular light-chain-associated amyloidogenesis depicts the crucial role of lysosomes. Kidney Int. 2014, 86, 738–746. [Google Scholar] [CrossRef]











| AL-Amyloidosis Cases | |||||||
|---|---|---|---|---|---|---|---|
| Case | Age | Sex | Pertinent Clinical Presentation | Proteinuria (gm/day) | Serum Creatinine (mg/dL) | Type of LC | Multiple Myeloma at Time of Biopsy |
| 1 | 73 | M | Sinus Tachycardia; Nephrotic syndrome | λ | No | ||
| 2 | 41 | F | Renal failure/Proteinuria | 12 | Ϗ | No | |
| 3 | 71 | F | Proteinuria; Atrial Fibrillation | 0.64-0.74 | λ | No | |
| 4 | 64 | F | Nephrotic syndrome | λ | Yes | ||
| 5 | 67 | M | Proteinuria | Ϗ | No | ||
| 6 | 70 | M | Proteinuria; CKD | 6 | 5.3 | λ | No |
| 7 | 64 | M | Renal failure/Proteinuria | 2–2.4 | Ϗ | Yes | |
| 8 | 71 | M | Nephrotic syndrome | 7 | λ | No | |
| 9 | 63 | M | Nephrotic syndrome | λ | Yes | ||
| 10 | 70 | F | Proteinuria; Renal insufficiency | 3.71 | λ | Yes | |
| 11 | 75 | M | Acute/Chronic kidney failure/Proteinuria | Ϗ | No | ||
| 12 | 71 | M | Waldenström macroglobulinemia/Renal failure | 4.5 | 1.7 | Ϗ | No |
| 13 | 65 | F | Proteinuria; Chronic lymphocytic leukemia | 13 | 0.83 | λ | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, G.A.; Teng, J.; Zeng, C.; Del Pozo-Yauner, L.; Liu, B.; Turbat-Herrera, E.A. Fibril-Forming Organelles in Mesangial Cells in Renal Biopsies from Patients with Light-Chain-Associated Amyloidosis. Hemato 2023, 4, 350-363. https://doi.org/10.3390/hemato4040028
Herrera GA, Teng J, Zeng C, Del Pozo-Yauner L, Liu B, Turbat-Herrera EA. Fibril-Forming Organelles in Mesangial Cells in Renal Biopsies from Patients with Light-Chain-Associated Amyloidosis. Hemato. 2023; 4(4):350-363. https://doi.org/10.3390/hemato4040028
Chicago/Turabian StyleHerrera, Guillermo A., Jiamin Teng, Chun Zeng, Luis Del Pozo-Yauner, Bing Liu, and Elba A. Turbat-Herrera. 2023. "Fibril-Forming Organelles in Mesangial Cells in Renal Biopsies from Patients with Light-Chain-Associated Amyloidosis" Hemato 4, no. 4: 350-363. https://doi.org/10.3390/hemato4040028