In addition to the Standard Deviation (SD), all samples have been characterized chemically, structurally and morphologically. The relevant parameters have been calculated for each of the applied techniques and the influence of each of the process variables has been evaluated individually by calculating the T-Student (5% confidence, reference value: 1.89) for each response. In this way, the influence of the variables on the parameters calculated for each technique can be established.
3.2.1. BET Analysis
The different GrO samples obtained were analyzed by BET, using the N
2 adsorption and desorption method to determine the SSA and t-Plot MV.
Table 3 shows the range, average, SD and T-Student of the results corresponding to SSA and t-Plot MV. The full results can be found in
Table A1, in
Appendix A.
The SSA data show an overall range of 23.4–67.0 (m
2g
−1). The maximum value obtained is higher than those obtained by other authors using the same synthesis [
89]. It is remarkable that the maximum value comes out at the shortest time.
The highest SSA average values have been obtained in the samples synthesized at 60 °C, 12 h, 50 mL H2O2 and 4 g KMnO4. Considering the calculation of the T-Student, it can be seen that the results obtained in the temperature variation are statistically discrepant. However, variations in the amounts of H2O2 and KMnO4 do not produce significant changes in the SSA of the obtained GrO.
In the case of t-Plot MV data, the overall range is between 0.0009–0.0181 (cm3g−1). The difference in the obtained results is of two orders of magnitude.
The average values of t-Plot MV show a similar variation to those obtained for SSA, the samples synthesized at 60 °C, 12 h, 50 mL H2O2 and 4 g KMnO4 being the ones with the highest t-Plot MV. According to the results obtained from T-Student, a significant discrepancy between the results obtained with the maximum and minimum temperature values is observed, as in the case of SSA, while variations in time and amounts of H2O2 and KMnO4 do not produce significant changes.
These results are in agreement with those obtained by ED, where the Pareto diagrams show that the variable that most affects SSA and t-Plot MV is temperature, and in this case, the results are also statistically discrepant.
Considering that most environmental and energy applications based on adsorption require high SSA and porosity, the best synthesis conditions would be at higher temperatures and reagent quantities and short times.
3.2.2. Elemental Analysis
All the samples obtained were analyzed to determine their total C content.
Table 4 shows the range, average, SD and T-Student of the results corresponding to carbon percentage.
The carbon percentage data show an overall range of 34.6–43.1 (%). The samples synthesized at a temperature of 45 °C present a slightly higher C content (average carbon content of 39.1%) than those synthesized at 60 °C (average: 37.8%). The highest % C average values were obtained in samples synthesized at 45 °C, 12 h, 10 mL H2O2 and 4 g KMnO4. It is observed that none of the values obtained for T-Student exceed the critical value, indicating that variations in temperature, time and amounts of H2O2 and KMnO4 do not produce significant changes in the % C of the GrO obtained.
These results are in agreement with those obtained by ED, where the Pareto diagrams (
Figure 4a) show that none of the experimental variables are statistically significant with respect to the % C values. The full results can be found in
Table A2 in
Appendix A.
In this case, the optimum % C depends on the intended application. GrO with lower % C could subsequently undergo different reduction treatments to recover the initial graphitic structure of the sheets and be used in applications requiring electrical conductivity, while more oxidized GrO are very versatile; thus, they can be chemically functionalized to improve their properties for selective adsorption applications.
3.2.3. XRD Analysis
The different GrO samples obtained after carrying out the 16 experiments of the ED have been analyzed by XRD to determine structural parameters. The XRD pattern of pure graphite presents a peak at 2θ = 26.58° (2Ɵ) corresponding to the [002] diffraction plane and an interplanar distance, d = 0.335 nm [
90]. In the oxidation process, the [002] diffraction plane broadens and decreases in intensity, while the [001] diffraction plane of GrO, appears at 2θ = 11° [
91]. This variation is due to the introduction of oxygenated groups, mainly hydroxy and epoxy on the surface of the individual graphene sheets, and carboxyl groups on the edges that break the crystalline structure. The presence of oxygenated groups increases the interplanar distance, distorting the initial structure of the stacked sheets.
Figure 5 shows the [001] diffraction plane corresponding to the GrO materials synthesized in the 16 experiments. The diffraction maxima of all GrO come out in the range of 9.83° < 2θ < 11.77°, corroborating that oxidation of graphite to GrO has occurred.
It can be seen that there is a clear difference between the samples synthesized at the two temperatures (the vertical black line marks an arbitrary division between the diffractograms of GrO synthesized at 45 °C and at 60 °C). The diffraction peaks corresponding to GrO synthetized at 45 °C (GrO_Exp02-06, GrO_08, GrO_12 and GrO_15) are narrower and more intense, while those synthesized at 60 °C (GrO_01, GrO_07, GrO_09-11, GrO_13, GrO_14, GrO_16) are less intense and wider. This could be due to the fact that at 45 °C, in this type of materials, gaseous CO
2 can start to be produced [
81], which could be adsorbed between the GrO films, and it can be desorbed and leave the material if there is high enough energy [
92], producing a certain degree of exfoliation and defects in the films, as mentioned previously in
Section 3.1. On the other hand, according to the literature, Mn
2O
7 decomposes at 55 °C and above [
82], generating bubbles and facilitating the separation of the GrO sheets. GrO samples synthesized at 60 °C are more affected by these phenomena, which produce distortion in the stacked structure, generate defects in the films, decrease crystallinity and cause exfoliation, giving rise to wider diffraction peaks of lower intensity. Additionally, the samples obtained at 45 °C show narrower and more intense diffraction peaks as they retain a greater proportion of stacked structure; therefore, the crystallinity of GrO is higher. This can be due to the fact that during their synthesis processes, they have been kept at the temperature at which CO
2 bubbles start to form, and most of them do not have enough energy to leave the material. These results are in agreement with those obtained in the ED, where the temperature is the most relevant parameter and statistically significant for SSA and t-Plot MV.
The average, SD and T-Student of the results corresponding to the 2θ positions, interplanar spacing (d), number of stacked layers (n) and crystal size (L) of the GrO obtained have been summarized in
Table 5 (the full results can be found in
Table A3 in
Appendix A). The number of stacked layers in the graphite oxide (n) was obtained by Equation (1), where “L” is the crystal size and “d” is the interplanar spacing:
Furthermore, L has been calculated using the Scherrer Equation (2) [
93]:
where λ is the X-ray wavelength, β is the line broadening at half the maximum and θ is the Bragg angle.
In
Table 5a, the average position and d data show an overall range of 9.8–11.2 (2Ɵ) and 0.79–0.90 (nm), respectively. It can be observed that the average values for position only show variations in the case of temperature changes, being statistically significant according to the obtained T-Student value (7.7). The same trend can be seen for d, for which a T-Student value of 7.5 has been calculated, exceeding the critical value only in the case of variations in the synthesis temperature.
On the other hand,
Table 5b shows the calculated values of n and L according to the formulae mentioned above (Equations (1) and (2)). The ranges of total average values for n and L are 27.7–166.1 (nm) and 33.7–197.7, respectively. For both cases, the T-Student exceeds the critical value only in the case of temperature variations, reaching a value of 4.5 in the case of L and 4.3 for n.
The general goal is to obtain more porous structures with higher SSA and GrO with smaller crystal sizes and a lower number of stacked layers. In this case, the best conditions are reached working at higher temperatures, since at 60 °C, an average L and n of 38.5 and 47.3 nm, respectively, are obtained, compared to 109.3 and 125.4 nm obtained at 45 °C.
According to
Table 5a,b, the GrO synthesized at 60 °C has a smaller average interplanar distance (0.81 nm) and a smaller average number of stacked layers [
47] compared to those synthesized at 45 °C (0.87 nm and 125), although the interplanar distance differences are slight.
This could be due, in part, to a slight accumulation of CO
2 bubbles, which starts to form at 45 °C, as described in previous sections. At this temperature, the gas bubbles do not yet have enough energy to leave the GrO structure, so they could be adsorbed between the sheets, generating pressure and increasing the interplanar spacing. On the other hand, at 60 °C, the CO
2 generation is higher, together with a higher oxidation of the graphite. At this temperature (60 °C), the CO
2 bubbles formed have enough energy to leave the GrO structure, which could result in smaller interplanar spacings than in the samples synthesized at 45 °C. However, the interplanar spacing in GrO depends on multiple factors in addition to this, such as the water content [
94,
95] and the number of stacked layers [
96], among others. Moreover, most of these factors are influenced by each other, such as CO
2 adsorption, which depends on the degree of hydration [
97], which, in turn, depends on the number of stacked layers [
96].
The increased production of CO
2 bubbles at higher temperatures also generates a higher degree of exfoliation and fragmentation in the materials synthesized at 60 °C. These effects result in fewer stacked layers and smaller crystal size (38.5 nm) in GrO synthesized at 60 °C compared to those obtained at 45 °C (109.3 nm), which is in agreement with the SEM images described below in
Section 3.2.5. This means that at higher temperatures, more graphitic surface area is exposed to the oxidant and oxidation occurs over a larger area. The higher degree of oxidation due to the exposure of more surface is corroborated by the results obtained by E.A, where lower % C are obtained for samples synthesized at 60 °C.
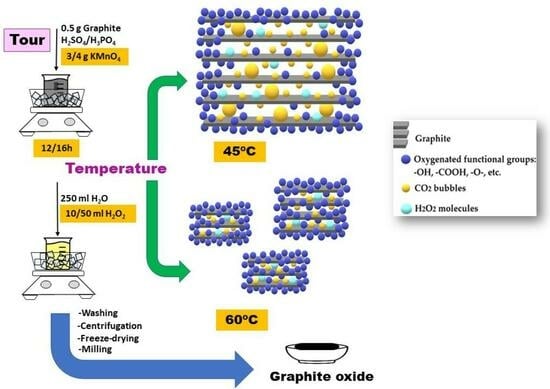
Figure 6 shows the possible GrO structure synthesized at 45 and 60 °C, taking into account all the results obtained.
3.2.4. Raman Analysis
In Raman spectroscopy, a typical spectrum corresponding to pure graphite presents three characteristic bands: G-band at ~1580 cm
−1, D-band at ~354 cm
−1 and 2D-band at ~2700 cm
−1 [
98]. The G band is the most intense and is associated with the graphite mode [
99]. It appears due to the first order dispersion of the E
2g
2 mode and is related to the number of sp
2 carbons. The D band is less intense and corresponds to the diamond mode. This band is attributed to disorder in the graphite, which may be due to amorphous carbon species, vacancies, defects in the structure, etc. [
100]. The ratio of intensities of the D and G bands (I
D/I
G) is used to estimate the density of defects present in the material studied and has a value of approximately 0.23 in pure graphite. In the case of GrO, defects appear mainly after the oxidation process, which incorporates oxygenated groups, generating sp
3 carbons and distorting the graphitic structure. Therefore, by the graphite oxidation, the D band increases in intensity due to the creation of defects, the incorporation of heteroatoms in the interplanar space and the increase of domains with sp
3 carbons. On the other hand, the intensity of the G band decreases because the domains of sp
2 carbons become smaller, until it becomes comparable to the D band. In the case of GrO, the I
D/I
G ratio grows to ~1.0 due to the incorporation of defects and sp
3 carbons after the oxidation process. The 2D band is related to the number of stacked graphene layers within the graphitic structure. The I
2D/I
G ratio, which is inversely proportional to the number of stacked layers, is often used to evaluate it. Values in the range 2–3 correspond to monolayers, values <1 correspond to multilayers, while intermediate values are considered as bilayers [
101].
The analysis of the 16 samples has provided the I
D/I
G ratios shown in
Figure 7 in order from highest to lowest I
D/I
G ratio, read from the bottom to the top of the graph. Data were generated by measuring each sample at three different points and obtaining their average value. It is observed that all values are in the range 0.896 < I
D/I
G < 1.033, indicating that defects and disorder have been generated in the structure during the oxidation process. There are no appreciable differences between the results of the samples synthesized at 45 °C and those obtained at 60 °C, but some influence of the amount of H
2O
2 is observed. Samples synthesized with 50 mL H
2O
2, such as GrO_Exp08, GrO_Exp05, GrO_Exp02, GrO_Exp11 and GrO_Exp14, show larger I
D/I
G values compared to others synthesized with 10 mL H
2O
2, such as GrO_Exp07, GrO_Exp04, GrO_Exp12, GrO_Exp03 and GrO_Exp16. This may be due to the exfoliation effect of H
2O mentioned above, which generates disorder in the GrO structure and thus exposes larger graphitic surfaces to oxidation, leading to higher amounts of defects.
Table 6 shows range, average, SD and T-Student of the results corresponding to the I
D/I
G and I
2D/I
G of the GrO obtained in the ED (the full results can be found in
Table A4 in
Appendix A). For I
D/I
G, a total range of average values of 0.90–1.03 can be observed. The highest T-Student is obtained for the case of temperature variations, with an I
D/I
G of 1.3; however, it does not exceed the critical value. On the other hand, the total range of average values for I
2D/I
G is between 0.13 and 0.18. According to the T-Student results, the variations of none of the studied variables are statistically significant for the I
D/I
G and I
2D/I
G ratios. The I
2D/I
G values obtained for both temperatures are very similar, indicating multilayer stacking in the GrO structure. This is in line with the XRD results discussed in the previous section (
Section 3.2.3), where the number of stacked layers are evaluated more accurately.
3.2.5. SEM Analysis
SEM analyses were also carried out to evaluate the morphology of the obtained GrO samples.
From the previous analysis, it can be reasoned that temperature is the most influential variable. Therefore, two samples have been selected (GrO_01 and GrO_05), in which the two different temperatures have been applied (45 and 60 °C), keeping the rest of the variables fixed (12 h, 50 mL H
2O
2 and 4 g KMnO
4) (
Figure 8). It is in these two cases where the greatest morphological differences can be seen.
In both samples, separate layers are observed, indicating partial exfoliation due to both the oxidation process and the freeze-drying process. The sample obtained at 60 °C (GrO_Exp01) shows a more disorganized structure and greater exfoliation, while in the sample obtained at 45 °C (GrO_Exp05), there are stacked layers with a certain degree of alignment, which is in agreement with the results obtained in XRD, where broader peaks were obtained at 60 °C. The most noticeable differences can be seen in
Figure 8d,h corresponding to the two syntheses. In these cases,
Figure 8d corresponding to the GrO_Exp01 synthesis, shows that the GrO sheets are smaller and more exfoliated and fragmented than in the GrO_Exp05 synthesis (
Figure 8h), partly due to the formation of CO
2 bubbles which, in this case, are released and produce these effects. This observation corroborates the scheme made in
Figure 6, which represents the possible structure of GrO obtained at the two temperatures studied thanks to the structural and morphological data provided by XRD, Raman and SEM techniques.