Soil Organic Carbon Depletion in Managed Temperate Forests: Two Case Studies from the Apennine Chain in the Emilia-Romagna Region (Northern Italy)
Abstract
:1. Introduction
2. Materials and Methods
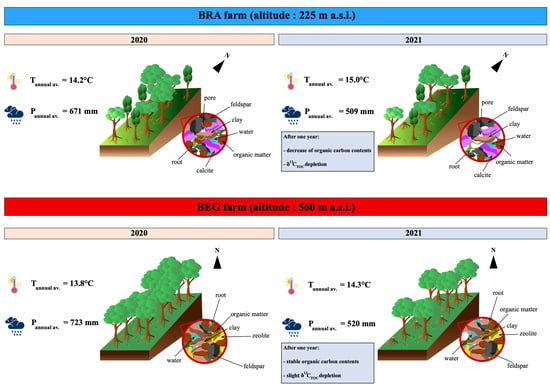
2.1. Sites Description
2.2. Sampling
2.3. Soil Sample Preparation, Textural Analysis, and Determination of Physicochemical Parameters
2.4. X-ray Powder Diffraction Data Collection
2.5. Geochemical Analyses
2.5.1. Carbon Elemental Analysis and Carbon Speciation
2.5.2. Carbon Isotopic Analysis
2.5.3. Nitrogen Elemental Analysis
2.6. Statistical Data Analysis
3. Results
3.1. Soil Texture and Physicochemical Properties
3.2. Phase Identification
3.3. Soil Carbon and Nitrogen Elemental Contents and Carbon Isotopic Ratios
4. Discussion
4.1. Variation in C and N Contents and C Isotopic Ratios in the BRA Soil over Time
4.2. Variation in C and N Contents and C Isotopic Ratios in BEG Soil over Time
4.3. Comparison between the Farms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banwart, S.A.; Nikolaidis, N.P.; Zhu, Y.-G.; Peacock, C.L.; Sparks, D.L. Soil Functions: Connecting Earth’s Critical Zone. Annu. Rev. Earth Planet. Sci. 2019, 47, 333–359. [Google Scholar] [CrossRef]
- Clunes, J.; Valle, S.; Dörner, J.; Martínez, O.; Pinochet, D.; Zúñiga, F.; Blum, W.E.H. Soil Fragility: A Concept to Ensure a Sustainable Use of Soils. Ecol. Indic. 2022, 139, 108969. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Sauer, T.J.; Cruse, R.M. Chapter One—Soil: The Forgotten Piece of the Water, Food, Energy Nexus. Adv. Agron. 2017, 143, 1–46. [Google Scholar]
- Haygarth, P.M.; Ritz, K. The Future of Soils and Land Use in the UK: Soil Systems for the Provision of Land-Based Ecosystem Services. Land Use Policy 2009, 26, 187–197. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of Forest Management Activities on Soil Organic Carbon Stocks: A Knowledge Synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Lal, R. Soil Management for Carbon Sequestration. S. Afr. J. Plant Soil 2021, 38, 231–237. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global Soil Carbon: Understanding and Managing the Largest Terrestrial Carbon Pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Santini, N.S.; Adame, M.F.; Nolan, R.H.; Miquelajauregui, Y.; Piñero, D.; Mastretta-Yanes, A.; Cuervo-Robayo, Á.P.; Eamus, D. Storage of Organic Carbon in the Soils of Mexican Temperate Forests. For. Ecol. Manag. 2019, 446, 115–125. [Google Scholar] [CrossRef]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest Carbon Management: A Review of Silvicultural Practices and Management Strategies across Boreal, Temperate and Tropical Forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Köhl, M.; Lasco, R.; Cifuentes, M.; Jonsson, Ö.; Korhonen, K.T.; Mundhenk, P.; de Jesus Navar, J.; Stinson, G. Changes in Forest Production, Biomass and Carbon: Results from the 2015 Un Fao Global Forest Resource Assessment. For. Ecol. Manag. 2015, 352, 21–34. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Y.; Peng, M.; Jin, Y.; Song, Q. Effects of Climate Change on the Carbon Sequestration Potential of Forest Vegetation in Yunnan Province, Southwest China. Forests 2022, 13, 306. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Kim, D.-G.; Li, J.; Liu, Y.; Hai, X.; Liu, Q.; Huang, C.; Shangguan, Z.; Kuzyakov, Y. Drought Effects on Soil Carbon and Nitrogen Dynamics in Global Natural Ecosystems. Earth Sci. Rev. 2021, 214, 103501. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Impacts of Climate Change on Natural Forest Productivity—Evidence since the Middle of the 20th Century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Blanco, J.A. Managing Forest Soils for Carbon Sequestration: Insights from Modeling Forests around the Globe. In Soil Management and Climate Change; Academic Press: Cambridge, MA, USA, 2018; pp. 237–252. [Google Scholar]
- Teng, F.-Z.; Ma, L. Deciphering Isotope Signatures of Earth Surface and Critical Zone Processes. Chem. Geol. 2016, 445, 1–3. [Google Scholar] [CrossRef]
- ARPAE. Rapporto Idrometeoclima Emilia-Romagna: Dati 2020; Arpae Emilia-Romagna: Bologna, Italy, 2021; p. 65. [Google Scholar]
- ARPAE. Rapporto Idrometeoclima Emilia-Romagna: Dati 2021; Arpae Emilia-Romagna: Bologna, Italy, 2022; p. 69. [Google Scholar]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff, Field Book for Describing and Sampling Soils, Version 3.0; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle Size Analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; ASA and SSSA: Madison, WI, USA, 1986; Volume 9. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and Gypsum. In Methods of Soil Analysis: Part 3 Chemical Methods; ASA and SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program Bgmn. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Zethof, J.H.T.; Leue, M.; Vogel, C.; Stoner, S.W.; Kalbitz, K. Identifying and Quantifying Geogenic Organic Carbon in Soils—The Case of Graphite. Soil 2019, 5, 383–398. [Google Scholar] [CrossRef]
- Natali, C.; Bianchini, G.; Carlino, P. Thermal Stability of Soil Carbon Pools: Inferences on Soil Nature and Evolution. Thermochim. Acta 2020, 683, 178478. [Google Scholar] [CrossRef]
- Natali, C.; Bianchini, G. Thermally Based Isotopic Speciation of Carbon in Complex Matrices: A Tool for Environmental Investigation. Environ. Sci. Pollut. Res. Int. 2015, 22, 12162–12173. [Google Scholar] [CrossRef]
- Natali, C.; Bianchini, G.; Vittori Antisari, L.; Natale, M.; Tessari, U. Carbon and Nitrogen Pools in Padanian Soils (Italy): Origin and Dynamics of Soil Organic Matter. Geochemistry 2018, 78, 490–499. [Google Scholar] [CrossRef]
- Gonfiantini, R.; Stichler, W.; Rozanski, K. Standards and Intercomparison Materials Distributed by the International Atomic Energy Agency for Stable Isotope Measurements, References and Intercomparison Materials for Stable Isotopes of Light Elements; IAEA: Vienna, Austria, 1993. [Google Scholar]
- Kusaka, S.; Nakano, T. Carbon and Oxygen Isotope Ratios and Their Temperature Dependence in Carbonate and Tooth Enamel Using a Gasbench II Preparation Device. Rapid Commun. Mass. Spectrom. 2014, 28, 563–567. [Google Scholar] [CrossRef]
- Beccaluva, L.; Bianchini, G.; Natali, C.; Siena, F. The Alkaline-Carbonatite Complex of Jacupiranga (Brazil): Magma Genesis and Mode of Emplacement. Gondwana Res. 2017, 44, 157–177. [Google Scholar] [CrossRef]
- Dutta, K.; Schuur, E.A.G.; Neff, J.C.; Zimov, S.A. Potential Carbon Release from Permafrost Soils of Northeastern Siberia. Glob. Chang. Biol. 2006, 12, 2336–2351. [Google Scholar] [CrossRef]
- Rstudio: Integrated Development Environment for R; R Studio, PBC: Boston, MA, USA, 2011; Available online: https://posit.co/download/rstudio-desktop/ (accessed on 22 June 2020).
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Harden, C.P. Soil Erosion and Sustainable Mountain Development. Mt. Res. Dev. 2001, 21, 77–83. [Google Scholar] [CrossRef]
- Stanchi, S.; Falsone, G.; Bonifacio, E. Soil Aggregation, Erodibility, and Erosion Rates in Mountain Soils (NW Alps, Italy). Solid Earth 2015, 6, 403–414. [Google Scholar] [CrossRef]
- Guerra, C.A.; Rosa, I.M.D.; Valentini, E.; Wolf, F.; Filipponi, F.; Karger, D.N.; Xuan, A.N.; Mathieu, J.; Lavelle, P.; Eisenhauer, N. Global Vulnerability of Soil Ecosystems to Erosion. Landsc. Ecol. 2020, 35, 823–842. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil Organic Carbon Storage as a Key Function of Soils—A Review of Drivers and Indicators at Various Scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Brombin, V.; Mistri, E.; De Feudis, M.; Forti, C.; Salani, G.M.; Natali, C.; Falsone, G.; Vittori Antisari, L.; Bianchini, G. Soil Carbon Investigation in Three Pedoclimatic and Agronomic Settings of Northern Italy. Sustainability 2020, 12, 10539. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Zhang, Q.; Cheng, X. Soil Carbon Dynamics Following Land-Use Change Varied with Temperature and Precipitation Gradients: Evidence from Stable Isotopes. Glob. Chang. Biol. 2015, 21, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, P.; Zhang, X.; Li, J.; Yu, Y.; Wan, Y.; Wang, Y.; Wang, X.; Liu, Z.; Pan, L.; et al. Transpiration Sensitivity to Drought in Quercus wutaishansea Mary Forests on Shady and Sunny Slopes in the Liupan Mountains, Northwestern China. Forests 2022, 13, 1999. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Hu, W.; Wang, L.; Li, Z.; Pan, J.; Chen, F. Stable Isotope Fractionation Provides Information on Carbon Dynamics in Soil Aggregates Subjected to Different Long-Term Fertilization Practices. Soil Tillage Res. 2018, 177, 54–60. [Google Scholar] [CrossRef]
- Hogberg, P. 15N Natural Abundance in Soil-Plant Systems. New Phytol. 2008, 137, 179–203. [Google Scholar] [CrossRef]
- Eshetu, E.Y.; Hailu, T.A.; Tejada Moral, M. Carbon Sequestration and Elevational Gradient: The Case of Yegof Mountain Natural Vegetation in North East, Ethiopia, Implications for Sustainable Management. Cogent Food Agric. 2020, 6, 1733331. [Google Scholar] [CrossRef]
- Moser, G.; Leuschner, C.; Hertel, D.; Graefe, S.; Soethe, N.; Iost, S. Elevation Effects on the Carbon Budget of Tropical Mountain Forests (S Ecuador): The Role of the Belowground Compartment. Glob. Chang. Biol. 2011, 17, 2211–2226. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Trumbore, S.E.; Vittori Antisari, L.; Cocco, S.; Corti, G.; Agnelli, A. Small Altitudinal Change and Rhizosphere Affect the SOM Light Fractions but Not the Heavy Fraction in European Beech Forest Soil. Catena 2019, 181, 104091. [Google Scholar] [CrossRef]
- Tian, Q.; He, H.; Cheng, W.; Bai, Z.; Wang, Y.; Zhang, X. Factors Controlling Soil Organic Carbon Stability Along a Temperate Forest Altitudinal Gradient. Sci. Rep. 2016, 6, 18783. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef]
- Calero, J.; García-Ruiz, R.; Torrús-Castillo, M.; Vicente-Vicente, J.L.; Martín-García, J.M. Role of Clay Mineralogy in the Stabilization of Soil Organic Carbon in Olive Groves under Contrasted Soil Management. Minerals 2023, 13, 60. [Google Scholar] [CrossRef]
- Islam, M.R.; Singh, B.; Dijkstra, F.A. Stabilisation of soil organic matter: Interactions between clay and microbes. Biogeochemistry 2022, 160, 145–158. [Google Scholar] [CrossRef]
- Singh, M.; Sarkar, B.; Sarkar, S.; Churchman, J.; Beerling, D.J. Stabilization of soil organic carbon as influenced by clay mineralogy. Adv. Agron. 2017, 148, 33–84. [Google Scholar]
- Sarkar, B.; Singh, M.; Mandal, S.; Churchman, G.J.; Bolan, N.S. Clay Minerals—Organic Matter Interactions in Relation to Carbon Stabilization in Soils. In The Future of Soil Carbon Its Conservation and Formation; Garcia, C., Nannipieri, P., Hernandez, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 71–86. [Google Scholar]
- Doni, S.; Gispert, M.; Peruzzi, E.; Macci, C.; Mattii, G.B.; Manzi, D.; Masini, C.M.; Grazia, M. Impact of Natural Zeolite on Chemical and Biochemical Properties of Vineyard Soils. Soil Use Manag. 2020, 37, 832–842. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Li, X.; Lu, J.; Gao, R.; Kamran, M.; Fahad, S. Effect of Clay Mineralogy and Soil Organic Carbon in Aggregates under Straw Incorporation. Agronomy 2022, 12, 534. [Google Scholar] [CrossRef]
- Rasmussen, C.; Heckman, K.; Wieder, W.R.; Keiluweit, M.; Lawrence, C.R.; Berhe, A.A.; Blankinship, J.C.; Crow, S.E.; Druhan, J.L.; Hicks Pries, C.E.; et al. Beyond clay: Towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018, 137, 297–306. [Google Scholar] [CrossRef]
- Kavvadias, V.; Ioannou, Z.; Vavoulidou, E.; Paschalidis, C. Short Term Effects of Chemical Fertilizer, Compost and Zeolite on Yield of Lettuce, Nutrient Composition and Soil Properties. Agriculture 2023, 13, 1022. [Google Scholar] [CrossRef]






| Site | Slope (%) |
|---|---|
| Branchicciolo forest | |
| BRA1 | 20 |
| BRA2 | 5 |
| BRA3 | 3 |
| BRA4 | 45 |
| Beghelli forest | |
| BEG1 | 20 |
| BEG2 | 45 |
| BEG3 | 32 |
| BEG4 | 45 |
| Site | Horizon | Depth (cm) | pH | EC (µS cm−1) | CaCO3 (g kg−1) | Sand (g kg−1) | Silt (g kg−1) | Clay (g kg−1) |
|---|---|---|---|---|---|---|---|---|
| Branchicciolo | ||||||||
| BRA 1 | Oi | 4–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| A1 | 0–5 | 7.6 | 426 | 107 | 827 | 168 | 5 | |
| A2 | 5–10.5 | 7.7 | 328 | 140 | 762 | 222 | 16 | |
| AB | 10.5–20.5 | 7.5 | 259 | 189 | 679 | 315 | 6 | |
| Bw | 20.5–33.5 | 7.1 | 200 | 229 | 703 | 231 | 66 | |
| BC | 33.5–40+ | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| BRA 2 | Oi | 4–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oe | 0–1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| A/Oe | 0–2/3 | 7.9 | 285 | 124 | 810 | 158 | 32 | |
| A | 2/3–11/12 | 7.8 | 293 | 166 | 684 | 252 | 64 | |
| Bw | 11/12–31 | 7.9 | 204 | 229 | 707 | 224 | 69 | |
| BC | 31–44 | 7.8 | 195 | 222 | 701 | 239 | 60 | |
| BRA 3 | Oi | 3–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oe | 0–2/3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| A | 2/3–16 | 7.6 | 280 | 173 | 729 | 210 | 61 | |
| Bw | 16–30 | 7.7 | 189 | 213 | 742 | 160 | 97 | |
| BC1 | 30–46/49 | 7.7 | 205 | 211 | 677 | 220 | 103 | |
| BC2 | 46/49–66+ | 7.9 | 195 | 226 | 651 | 234 | 115 | |
| BRA 4 | Oe | 0–1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| A | 1–2 | 7.6 | 364 | 56 | 767 | 203 | 29 | |
| AB | 2–7 | 7.4 | 272 | 127 | 645 | 276 | 79 | |
| Bw1 | 7–15 | 7.6 | 255 | 144 | 677 | 246 | 77 | |
| Bw2 | 15–27 | 7.8 | 222 | 167 | 586 | 309 | 105 | |
| BC | 27–37 | 7.8 | 213 | 178 | 555 | 342 | 103 | |
| Beghelli | ||||||||
| BEG 1 | Oi | 2–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oe | 0–1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| A | 1–7 | 6.9 | 258 | 111 | 507 | 434 | 59 | |
| Bw | 7–24 | 7.1 | 298 | 36 | 370 | 355 | 275 | |
| BC | 24–37 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| BEG 2 | Oi | 0.2–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| A1 | 0–4/5 | 5.6 | 191 | 0 | 582 | 337 | 81 | |
| A2 | 4/5–6/7 | 5.4 | 99 | 0 | 460 | 399 | 141 | |
| Bw | 6/7–21 | 4.9 | 70 | 0 | 315 | 450 | 235 | |
| BC1 | 21–36 | 5.9 | 89 | 0 | 334 | 383 | 283 | |
| BC2 | 36–51 | 5.7 | 98 | 0 | 347 | 258 | 395 | |
| C | 51–61+ | 7.4 | 180 | 0 | 504 | 180 | 316 | |
| BEG 3 | Oi | 0.5–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Oe/Oa | 0–0.5 | 6.8 | 311 | 182 | n.d. | n.d. | n.d. | |
| AE | 0.5–4.5/7.5 | 6.3 | 150 | 0 | 294 | 468 | 238 | |
| AB | 4.5/7.5–23 | 6.9 | 279 | 0 | 255 | 500 | 245 | |
| Bw | 23–38 | 7.4 | 324 | 0 | 225 | 458 | 317 | |
| BC1 | 38–45 | 7.5 | 225 | 0 | 203 | 435 | 362 | |
| BC2 | 45–65+ | 7.0 | 144 | 0 | 235 | 439 | 326 | |
| BEG 4 | Oi | 2–0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| A | 0–7 | 6.8 | 253 | 0 | 534 | 371 | 95 | |
| Bw1 | 7–18 | 6.3 | 58 | 0 | 214 | 532 | 254 | |
| Bw2 | 18–38 | 7.2 | 154 | 0 | 294 | 464 | 242 | |
| C | 38–49 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Farm | Branchicciolo | Beghelli | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | BRA 1 | BRA 2 | BRA 3 | BRA 4 | BEG 2 | BEG 3 | BEG 4 | |||||||
| wt% | wt% | wt% | wt% | wt% | wt% | wt% | ||||||||
| Quartz | 29.5 | ±0.4 | 33.4 | ±0.3 | 34.0 | ±0.3 | 33.9 | ±0.3 | 28.5 | ±0.3 | 30.7 | ±0.4 | 40.5 | ±0.4 |
| Feldspars | ||||||||||||||
| Albite | 8.3 | ±0.3 | 9.5 | ±0.3 | 11.5 | ±0.3 | 9.6 | ±0.3 | 9.4 | ±0.3 | 11.5 | ±0.3 | 13.5 | ±0.3 |
| Microcline | 8.0 | ±0.6 | 4.2 | ±0.3 | 6.8 | ±0.4 | 4.7 | ±0.4 | 9.9 | ±0.4 | 9.0 | ±0.5 | 10.5 | ±0.5 |
| Phyllosilicates | ||||||||||||||
| Muscovite | 6.8 | ±0.6 | 8.6 | ±0.5 | 11.6 | ±0.6 | 12.7 | ±0.6 | 20.5 | ±0.5 | 21.0 | ±0.6 | 4.3 | ±0.5 |
| Paragonite | --- | --- | --- | --- | --- | --- | --- | --- | 6.3 | ±0.3 | --- | --- | 7.9 | ±0.4 |
| Kaolinite | 3.5 | ±0.2 | 5.2 | ±0.4 | 8.0 | ±0.4 | 6.1 | ±0.4 | 15.5 | ±0.4 | 13.2 | ±0.5 | 6.6 | ±0.3 |
| Illite | 5.0 | ±0.7 | 8.4 | ±0.5 | 2.9 | ±0.6 | 6.7 | ±0.6 | 3.5 | ±0.5 | 7.0 | ±0.6 | 6.7 | ±0.6 |
| Chlorite | 9.1 | ±0.7 | 6.4 | ±0.5 | 4.7 | ±0.4 | 4.8 | ±0.4 | --- | --- | --- | --- | 4.3 | ±0.1 |
| Vermiculite | --- | --- | --- | --- | --- | --- | --- | --- | 2.1 | ±0.1 | 1.6 | ±0.1 | 0.6 | ±0.1 |
| Carbonates | ||||||||||||||
| Calcite | 29.8 | ±0.4 | 24.3 | ±0.2 | 20.4 | ±0.3 | 21.5 | ±0.2 | --- | --- | --- | --- | --- | --- |
| Zeolites | ||||||||||||||
| Clinoptilolite | --- | --- | --- | --- | --- | --- | --- | --- | 4.4 | ±0.2 | 6.0 | ±0.3 | 5.0 | ±0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brombin, V.; Salani, G.M.; De Feudis, M.; Mistri, E.; Precisvalle, N.; Bianchini, G. Soil Organic Carbon Depletion in Managed Temperate Forests: Two Case Studies from the Apennine Chain in the Emilia-Romagna Region (Northern Italy). Environments 2023, 10, 156. https://doi.org/10.3390/environments10090156
Brombin V, Salani GM, De Feudis M, Mistri E, Precisvalle N, Bianchini G. Soil Organic Carbon Depletion in Managed Temperate Forests: Two Case Studies from the Apennine Chain in the Emilia-Romagna Region (Northern Italy). Environments. 2023; 10(9):156. https://doi.org/10.3390/environments10090156
Chicago/Turabian StyleBrombin, Valentina, Gian Marco Salani, Mauro De Feudis, Enrico Mistri, Nicola Precisvalle, and Gianluca Bianchini. 2023. "Soil Organic Carbon Depletion in Managed Temperate Forests: Two Case Studies from the Apennine Chain in the Emilia-Romagna Region (Northern Italy)" Environments 10, no. 9: 156. https://doi.org/10.3390/environments10090156