Novel Fenton-like Catalyst HKUST-1(Cu)/MoS2-3-C with Non-Equilibrium-State Surface for Selective Degradation of Phenolic Contaminants: Synergistic Effects of σ-Cu-Ligand and ≡Mo–OOSO3− Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Catalysts
2.3. Characterization
2.4. Catalytic Degradation Experiments
3. Results and Discussion
3.1. Morphology and Structure of Catalysts
3.2. Catalytic Performance
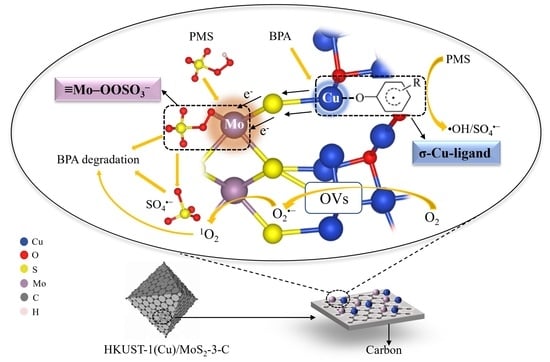
3.3. Catalytic Mechanism


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lyu, L.; Zhang, L.; Wang, Q.; Nie, Y.; Hu, C. Enhanced Fenton Catalytic Efficiency of gamma-Cu-Al2O3 by σ-Cu2+-Ligand Complexes from Aromatic Pollutant Degradation. Environ. Sci. Technol. 2015, 49, 8639–8647. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Brillas, E.; Sires, I. Finding the best Fe2+/Cu2+ combination for the solar photoelectro-Fenton treatment of simulated wastewater containing the industrial textile dye Disperse Blue 3. Appl. Catal. B Environ. 2012, 115, 107–116. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croué, J.-P. Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Cheng, M.M.; Ma, J.H.; Ma, W.H.; Chen, C.C.; Zhao, J.C. Decomposition of hydrogen peroxide driven by photochemical cycling of iron species in clay. Environ. Sci. Technol. 2006, 40, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Yan, D.; Yu, G.; Cao, W.; Hu, C. Efficient Destruction of Pollutants in Water by a Dual-Reaction-Center Fenton-like Process over Carbon Nitride Compounds-Complexed Cu(II)-CuAlO2. Environ. Sci. Technol. 2018, 52, 4294–4304. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Zhang, L.; He, G.; He, H.; Hu, C. Selective H2O2 conversion to hydroxyl radicals in the electron-rich area of hydroxylated C-g-C3N4/CuCo–Al2O3. J. Mater. Chem. A 2017, 5, 7153–7164. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, H.; Cao, W.; Wen, Z.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Cu-Al2O3-g-C3N4 and Cu-Al2O3-C-dots with dual-reaction centres for simultaneous enhancement of Fenton-like catalytic activity and selective H2O2 conversion to hydroxyl radicals. Appl. Catal. B Environ. 2018, 234, 223–233. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, L.; Hu, C. Galvanic-like cells produced by negative charge nonuniformity of lattice oxygen on d-TiCuAl–SiO2 nanospheres for enhancement of Fenton-catalytic efficiency. Environ. Sci. Nano 2016, 3, 1483–1492. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.J.; Gong, Y.; Zhu, Y.; Yang, Z.; Li, B.; Lu, Q.; Yu, Y.; Han, S.; Zhang, Z.; et al. Edge Epitaxy of Two-Dimensional MoSe2 and MoS2 Nanosheets on One-Dimensional Nanowires. J. Am. Chem. Soc. 2017, 139, 8653–8660. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Zhang, S.; Hua, M.; Qian, J.; Pan, B. Revisiting the Heterogeneous Peroxymonosulfate Activation by MoS2: A Surface Mo–Peroxymonosulfate Complex as the Major Reactive Species. ACS EST Water 2022, 2, 376–384. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.-y.; Zhou, Y.; Gao, Y.; Guan, C.; Jiang, J. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment. Chem. Eng. J. 2019, 371, 842–847. [Google Scholar] [CrossRef]
- Yang, L.; Chen, W.; Huang, J.; Tang, X.; Yang, R.; Zhang, H.; Tang, Z.; Gui, X. Resistance Switching and Failure Behavior of the MoOx/Mo2C Heterostructure. ACS Appl. Mater. Interfaces 2021, 13, 41857–41865. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kim, H.-K.; Liu, Y.; Li, W.; Griffiths, J.T.; Wu, Y.; Laha, S.; Fong, K.D.; Podjaski, F.; Yun, C.; et al. Bottom-up Formation of Carbon-Based Structures with Multilevel Hierarchy from MOF–Guest Polyhedra. J. Am. Chem. Soc. 2018, 140, 6130–6136. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, Y.; Xu, G.; Tian, Y.; Ma, D.; Zhang, Y.; Qiu, P. Synthesis of Multilevel Structured MoS2@Cu/Cu2O@C Visible-Light-Driven Photocatalyst Derived from MOF–Guest Polyhedra for Cyclohexane Oxidation. ACS Sustain. Chem. Eng. 2020, 8, 6622–6633. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Dubot, P.; Jousset, D.; Pinet, V.; Pellerin, F.; Langeron, J.P. Simulation of the LMM Auger spectra of copper. Surf. Interface Anal. 1988, 12, 99–104. [Google Scholar] [CrossRef]
- Zhu, G.; Shi, C.; Jin, Y.; Ge, M. Enhanced degradation of polyvinyl alcohol over a Cu0-carbon@γ-Al2O3 composite through heterogeneous Fenton-like reactions: Preparation, mechanism, and applications. Appl. Catal. A Gen. 2022, 643, 118721. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Aleisa, R.; Zhao, T.; Ma, S.; Zhang, G.; Yao, T.; Yin, Y. MoS2/FeS Nanocomposite Catalyst for Efficient Fenton Reaction. ACS Appl. Mater. Interfaces 2021, 13, 51829–51838. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Zhou, Y. Efficiently activate peroxymonosulfate by Fe3O4@MoS2 for rapid degradation of sulfonamides. Chem. Eng. J. 2021, 422, 130126. [Google Scholar] [CrossRef]
- Nong, Y.; Zhang, M.; Li, Q.; Pan, Q.; Huang, Y.; Wang, H.; Zheng, F.; Li, Q. Carbon coated bimetallic sulfides Co9S8/ZnS heterostructures microrods as advanced anode materials for lithium ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 141, 104601. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, C.; Wu, C.; Li, X.; Jin, M.; Lan, W.; Pan, X.; Zhou, J.; Xie, E. Cobalt sulfide embedded carbon nanofibers as a self-supporting template to improve lithium ion battery performances. Electrochim. Acta 2021, 366, 137351. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Liu, H.; Qu, J. Confining Free Radicals in Close Vicinity to Contaminants Enables Ultrafast Fenton-like Processes in the Interspacing of MoS2 Membranes. Angew. Chem. Int. Ed. 2019, 58, 8134–8138. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 Nanosheets with Widened Interlayer Spacing for High-Efficiency Removal of Mercury in Aquatic Systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Bai, R.; Yan, W.; Xiao, Y.; Wang, S.; Tian, X.; Li, J.; Xiao, X.; Lu, X.; Zhao, F. Acceleration of peroxymonosulfate decomposition by a magnetic MoS2/CuFe2O4 heterogeneous catalyst for rapid degradation of fluoxetine. Chem. Eng. J. 2020, 397, 125501. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, J.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Novel Fenton-like catalyst γ-Cu-Al2O3-Bi12O15Cl6 with electron-poor Cu centre and electron-rich Bi centre for enhancement of phenolic compounds degradation and H2O2 utilization: The synergistic effects of σ-Cu-ligand, dual-reaction centres and oxygen vacancies. Appl. Catal. B Environ. 2019, 253, 28–40. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Wang, Z.; Bush, R.T.; Sullivan, L.A.; Chen, C.; Liu, J. Selective oxidation of arsenite by peroxymonosulfate with high utilization efficiency of oxidant. Environ. Sci. Technol. 2014, 48, 3978–3985. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Strategic combination of N-doped graphene and g-C3N4: Efficient catalytic peroxymonosulfate-based oxidation of organic pollutants by non-radical-dominated processes. Appl. Catal. B Environ. 2020, 272, 119005. [Google Scholar] [CrossRef]
- Lyu, Z.; Xu, M.; Wang, J.; Li, A.; François-Xavier Corvini, P. Hierarchical nano-vesicles with bimetal-encapsulated for peroxymonosulfate activation: Singlet oxygen-dominated oxidation process. Chem. Eng. J. 2022, 433, 133581. [Google Scholar] [CrossRef]
- Oess, A.C.; Cheshire, M.V.; McPhail, D.B.; Stoll, S.; Alailia, M.E.; Vedy, J.C. Elucidation of phenol-Cu interaction mechanisms by potentiometry, ESR, UV absorption spectroscopy and molecular simulations. Sci. Total Environ. 1999, 228, 49–58. [Google Scholar] [CrossRef]
- Han, J.; Du, Z.; Zou, W.; Li, H.; Zhang, C. In-situ improved phenol adsorption at ions-enrichment interface of porous adsorbent for simultaneous removal of copper ions and phenol. Chem. Eng. J. 2015, 262, 571–578. [Google Scholar] [CrossRef]
- Mitić, Ž.; Nikolić, G.S.; Cakić, M.; Premović, P.; Ilić, L. FTIR spectroscopic characterization of Cu(II) coordination compounds with exopolysaccharide pullulan and its derivatives. J. Mol. Struct. 2009, 924–926, 264–273. [Google Scholar] [CrossRef]
- Zuo, S.; Guan, Z.; Xia, D.; Yang, F.; Xu, H.; Huang, M.; Li, D. Polarized heterogeneous CuO-CN for peroxymonosulfate nonradical activation: An enhancement mechanism of mediated electron transfer. Chem. Eng. J. 2020, 420, 127619. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Liu, C.; Lan, H.; Liu, H.; Qu, J. Interface Stabilization of Undercoordinated Iron Centers on Manganese Oxides for Nature-Inspired Peroxide Activation. ACS Catal. 2018, 8, 1090–1096. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Yin, Z.; Liu, Z.; Liu, Y.; Yang, Z.; Yang, W. Adsorption and catalysis of peroxymonosulfate on carbocatalysts for phenol degradation: The role of pyrrolic-nitrogen. Appl. Catal. B Environ. 2022, 319, 121891. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Ma, J.; Tian, H.; Qiang, Z. Surface hydroxyl groups of synthetic α-FeOOH in promoting OH generation from aqueous ozone: Property and activity relationship. Appl. Catal. B Environ. 2008, 82, 131–137. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D.; Zhao, G. Facile Construction of a Copper-Containing Covalent Bond for Peroxymonosulfate Activation: Efficient Redox Behavior of Copper Species via Electron Transfer Regulation. ACS Appl. Mater. Interfaces 2020, 12, 42790–42802. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, Z.; Ye, Z.; He, J.; Zheng, Z.; Gong, X.; Zhang, J.; Lo, I.M.C. Superoxide radicals dominated visible light driven peroxymonosulfate activation using molybdenum selenide (MoSe2) for boosting catalytic degradation of pharmaceuticals and personal care products. Appl. Catal. B Environ. 2021, 296, 120223. [Google Scholar] [CrossRef]
- Yi, Q.; Ji, J.; Shen, B.; Dong, C.; Liu, J.; Zhang, J.; Xing, M. Singlet Oxygen Triggered by Superoxide Radicals in a Molybdenum Cocatalytic Fenton Reaction with Enhanced REDOX Activity in the Environment. Environ. Sci. Technol. 2019, 53, 9725–9733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, S.; Fan, Z.; Wu, H.; Liu, F.; Chen, Z.; Li, A. Confinement of CoP Nanoparticles in Nitrogen-Doped Yolk-Shell Porous Carbon Polyhedron for Ultrafast Catalytic Oxidation. Adv. Funct. Mater. 2020, 30, 2003947. [Google Scholar] [CrossRef]
- Duan, P.; Qi, Y.; Feng, S.; Peng, X.; Wang, W.; Yue, Y.; Shang, Y.; Li, Y.; Gao, B.; Xu, X. Enhanced degradation of clothianidin in peroxymonosulfate/catalyst system via core-shell FeMn@N-C and phosphate surrounding. Appl. Catal. B Environ. 2020, 267, 118717. [Google Scholar] [CrossRef]
- Huang, G.X.; Wang, C.Y.; Yang, C.W.; Guo, P.C.; Yu, H.Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Jiang, X.H.; Zhong, Z.A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon Nitride Supported High-Loading Fe Single-Atom Catalyst for Activation of Peroxymonosulfate to Generate 1O2 with 100% Selectivity. Angew. Chem. Int. Ed. Engl. 2021, 60, 21751–21755. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lyu, Z.; Wang, J.; Li, A.; Corvini, P.F.X. Ultrafine-Mn2O3@N-doped porous carbon hybrids derived from Mn-MOFs: Dual-reaction centre catalyst with singlet oxygen-dominant oxidation process. Chem. Eng. J. 2022, 429, 132299. [Google Scholar] [CrossRef]
- Wu, L.; Sun, Z.; Zhen, Y.; Zhu, S.; Yang, C.; Lu, J.; Tian, Y.; Zhong, D.; Ma, J. Oxygen Vacancy-Induced Nonradical Degradation of Organics: Critical Trigger of Oxygen (O2) in the Fe-Co LDH/Peroxymonosulfate System. Environ. Sci. Technol. 2021, 55, 15400–15411. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Wang, Y.; Huang, J.; Wang, W.; Duan, L.; Yang, X.; Yu, X.; Han, X.; Liu, N. Nonradical activation of peroxydisulfate promoted by oxygen vacancy-laden NiO for catalytic phenol oxidative polymerization. Appl. Catal. B Environ. 2019, 254, 166–173. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Xie, L.; Li, Y.; Liu, Y.; Sun, Y.; Bu, Y.; Mi, X.; Zhan, S.; Hu, W. Prominent role of oxygen vacancy for superoxide radical and hydroxyl radical formation to promote electro-Fenton like reaction by W-doped CeO2 composites. Appl. Surf. Sci. 2021, 549, 149262. [Google Scholar] [CrossRef]
- Pan, Y.; Su, H.; Zhu, Y.; Vafaei Molamahmood, H.; Long, M. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Res. 2018, 145, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, Y.; Zhou, W. Cu-doped Ni-LDH with abundant oxygen vacancies for enhanced methyl 4-hydroxybenzoate degradation via peroxymonosulfate activation: Key role of superoxide radicals. J. Colloid Interface Sci. 2022, 610, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of Peroxymonosulfate by Oxygen Vacancies-Enriched Cobalt-Doped Black TiO2 Nanotubes for the Removal of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef]
- Qin, W.; Fang, G.; Wang, Y.; Zhou, D. Mechanistic understanding of polychlorinated biphenyls degradation by peroxymonosulfate activated with CuFe2O4 nanoparticles: Key role of superoxide radicals. Chem. Eng. J. 2018, 348, 526–534. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Qian, J.; Pan, B. Are Free Radicals the Primary Reactive Species in Co(II)-Mediated Activation of Peroxymonosulfate? New Evidence for the Role of the Co(II)-Peroxymonosulfate Complex. Environ. Sci. Technol. 2021, 55, 6397–6406. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Li, H.; Qian, J. Degradation of organic contaminants in the CoFe2O4/peroxymonosulfate process: The overlooked role of Co(II)-PMS complex. Chem. Eng. J. Adv. 2021, 8, 100143. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Yin, H.; Wang, R.; Wang, J.; Li, A. Novel Fenton-like Catalyst HKUST-1(Cu)/MoS2-3-C with Non-Equilibrium-State Surface for Selective Degradation of Phenolic Contaminants: Synergistic Effects of σ-Cu-Ligand and ≡Mo–OOSO3− Complex. Water 2024, 16, 121. https://doi.org/10.3390/w16010121
Yin X, Yin H, Wang R, Wang J, Li A. Novel Fenton-like Catalyst HKUST-1(Cu)/MoS2-3-C with Non-Equilibrium-State Surface for Selective Degradation of Phenolic Contaminants: Synergistic Effects of σ-Cu-Ligand and ≡Mo–OOSO3− Complex. Water. 2024; 16(1):121. https://doi.org/10.3390/w16010121
Chicago/Turabian StyleYin, Xiaoze, Huaqin Yin, Renjie Wang, Jinnan Wang, and Aimin Li. 2024. "Novel Fenton-like Catalyst HKUST-1(Cu)/MoS2-3-C with Non-Equilibrium-State Surface for Selective Degradation of Phenolic Contaminants: Synergistic Effects of σ-Cu-Ligand and ≡Mo–OOSO3− Complex" Water 16, no. 1: 121. https://doi.org/10.3390/w16010121