Enhanced Photocatalytic Ozonation of Phenol by Ag/ZnO Nanocomposites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.2. Activity
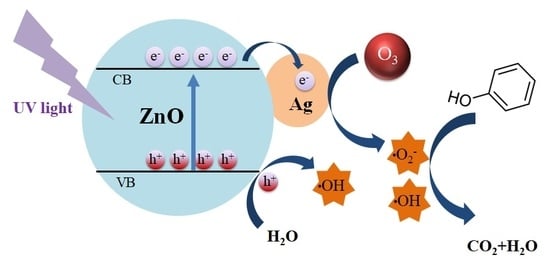
2.3. Reaction Mechanism
3. Materials and Methods
3.1. Catalyst Preparation and Characterization
3.2. Photocatalytic Ozonation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Agustina, T.E.; Ang, H.M.; Vareek, V.K. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 6, 264–273. [Google Scholar] [CrossRef]
- Xiao, J.D.; Xie, Y.B.; Cao, H.B. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mehrjouei, M.; Muller, S.; Moller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Pan, Z.H.; Cai, Q.H.; Luo, Q.; Li, X.W. Mechanism and kinetics of H-acid degradation in TiO2/O3/UV process. Can. J. Chem. Eng. 2014, 92, 851–860. [Google Scholar] [CrossRef]
- Marque, G.; Rodriguez, E.M.; Maldonado, M.I.; Alvarez, P.M. Integration of ozone and solar TiO2-photocatalytic oxidation for the degradation of selected pharmaceutical compounds in water and wastewater. Sep. Purif. Technol. 2014, 136, 18–26. [Google Scholar] [CrossRef]
- Liu, X.L.; Guo, Z.; Zhou, L.B.; Yang, J.; Cao, H.B.; Xiong, M.; Xie, Y.B.; Jia, G.R. Hierarchical biomimetic BiVO4 for the treatment of pharmaceutical wastewater in visible-light photocatalytic ozonation. Chemosphere 2019, 222, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, J.D.; Cao, H.B.; Guo, Z.; Rabeah, J.; Bruckner, A.; Xie, Y.B. The role of ozone and influence of band structure in WO3 photocatalysis and ozone integrated process for pharmaceutical wastewater treatment. J. Hazard. Mater. 2018, 360, 481–489. [Google Scholar] [CrossRef]
- Rey, A.; Mena, E.; Chavez, A.M.; Beltran, F.J.; Medina, F. Photocatalytic water treatment over WO3 under visible light irradiation combined with ozonation. Chem. Phys. Lett. 2010, 500, 86–89. [Google Scholar]
- Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Water treatment efficacy of various metal oxide semiconductors for photocatalytic ozonation under UV and visible light irradiation. Chem. Eng. J. 2015, 264, 221–229. [Google Scholar] [CrossRef]
- Ling, Y.; Liao, G.Z.; Xu, P.; Li, L.S. Fast mineralization of acetaminophen by highly dispersed Ag/g-C3N4 hybrid assisted photocatalytic ozonation. Sep. Purif. Technol. 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Yin, J.; Liao, G.Z.; Zhou, J.L.; Huang, C.M.; Ling, Y.; Lu, P.; Li, L.S. High performance of magnetic BiFeO3 nanoparticle-mediated photocatalytic ozonation for wastewater decontamination. Sep. Purif. Technol. 2016, 168, 134–140. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Orge, C.A.; Soares, O.; Ramalho, P.S.F.; Pereira, M.F.R.; Faria, J.L.; Salomé, G.P. Magnetic nanoparticles for photocatalytic ozonation of organic pollutants. Catalysts 2019, 9, 703. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, Z.L.; Zhao, S.Q.; He, W.; Yang, L. Enhanced ozonation of dichloroacetic acid in aqueous solution using nanometer ZnO powders. J. Environ. Sci. 2010, 22, 1527–1533. [Google Scholar] [CrossRef]
- Yuan, X.J.; Yan, X.; Xu, H.M.; Li, D.Y.; Sun, L.; Cao, G.; Xia, D.S. Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: Performance, kinetics and effects. J. Environ. Sci. 2017, 61, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Su, T.M.; Jiang, Y.X.; Xie, X.L.; Qin, Z.Z.; Ji, H.B. In situ DRIFTS study of O3 adsorption on CaO, gamma-Al2O3, CuO, alpha-Fe2O3 and ZnO at room temperature for the catalytic ozonation of cinnamaldehyde. Appl. Surf. Sci. 2019, 412, 290–305. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, Q.F.; Tian, S.Q.; Zhao, X.J. Exposed facet dependent stability of ZnO micro/nano crystals as a photocatalyst. Appl. Surf. Sci. 2019, 470, 807–816. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalin. Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Jing, L.Q.; Zhou, W.; Tian, G.H.; Fu, H.G. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef]
- Jing, L.Q.; Wang, D.J.; Wang, B.Q.; Li, S.D.; Xin, B.F.; Fu, H.G.; Sun, J.Z. Effects of noble metal modification on surface oxygen composition, charge separation and photocatalytic activity of ZnO nanoparticles. J. Mol. Catal. A: Chem. 2006, 244, 193–200. [Google Scholar]
- Qi, K.Z.; Cheng, B.; Yu, J.G.; Ho, W.K. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Liu, X.Q.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.H.; Zhao, S.Q.; Li, Z.; Lin, Z.Q. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Wu, A.P.; Tian, C.G.; Yan, H.J.; Hong, Y.; Jiang, B.J.; Fu, H.G. Intermittent microwave heating-promoted rapid fabrication of sheet-like Ag assemblies and small-sized Ag particles and their use as co-catalyst of ZnO for enhanced photocatalysis. J. Mater. Chem. A 2014, 2, 3015–3023. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.Z.; Sun, W.; Huang, J.C.; Xie, H.; Zhao, X.J. Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. J. Photochem. Photobiol. A 2010, 216, 149–155. [Google Scholar] [CrossRef]
- Raji, R.; Sibi, K.S.; Gopchandran, K.G. ZnO: Ag nanorods as efficient photocatalysts: Sunlight driven photocatalytic degradation of sulforhodamine B. Appl. Surf. Sci. 2019, 427, 863–875. [Google Scholar]
- Zhua, X.L.; Liang, X.H.; Wang, P.; Dai, Y.; Huang, B.B. Porous Ag-ZnO microspheres as efficient photocatalyst for methane and ethylene oxidation: Insight into the role of Ag particles. Appl. Surf. Sci. 2018, 456, 493–500. [Google Scholar] [CrossRef]
- Wang, R.P.; Xu, G.; Jin, P. Size dependence of electron-phonon coupling in ZnO nanowires. Phys. Rev. B 2004, 69, 13303–13304. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Xu, H.; Chen, Y.; Shen, X.; Xu, Z. Photochemical deposition of Ag nanocrystals on hierarchical ZnO microspheres and their enhanced gas-sensing properties. CrystEngComm 2012, 14, 719–725. [Google Scholar] [CrossRef]
- Cacciato, G.; Bayle, M.; Pugliara, A.; Bonafos, C.; Zimbone, M.; Privitera, V.; Grimaldi, M.G.; Carles, R. Enhancing carrier generation in TiO2 by a synergistic effect between plasmon resonance in Ag nanoparticles and optical interference. Nanoscale 2015, 7, 13468–13476. [Google Scholar] [CrossRef]
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhanga, Q.P.; Xua, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.Q.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Zheng, L.R.; Zhan, Y.Y.; Lin, X.Y.; Zheng, Q.; Wei, K.M. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gao, S.; Wang, J. One-pot synthesis of Ag/ZnO self-assembled 3D hollow microspheres with enhanced photocatalytic performance. J. Phys. Chem. C 2008, 112, 16792–16800. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Chen, N.; Xing, X.X.; Dong, C.J.; Wang, Y.D. Ag-ZnO heterostructure nanoparticles with plasmon-enhanced catalytic degradation for Congo red under visible light. RSC Adv. 2015, 5, 34456–34465. [Google Scholar] [CrossRef]
- Lai, Y.; Meng, M.; Yu, Y. One-step synthesis, characterizations and mechanistic study of nanosheets-constructed fluffy ZnO and Ag/ZnO spheres used for Rhodamine B photodegradation. Appl. Catal. B: Environ. 2010, 100, 491–501. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Murcia, J.J.; Rojas, H.; Navío, J.A.; Hidalgo, M.C. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Appl. Catal. B: Environ. 2018, 225, 197–206. [Google Scholar] [CrossRef]
- Hasabeldaim, E.H.H.; Ntwaeaborwa, O.M.; Kroon, R.E.; Coetsee, E.; Swart, H.C. Enhanced green luminescence from ZnO nanorods. J. Vac. Sci. Technol. B 2019, 37, 011201. [Google Scholar] [CrossRef]
- Nouri, H.; Habibi-Yangjeh, A.; Azadi, M. Preparation of Ag/ZnMgO nanocomposites as novel highly efficient photocatalysts by one-pot method under microwave irradiation. J. Photochem. Photobiol. A 2014, 281, 59–67. [Google Scholar] [CrossRef]
- Huang, M.L.; Weng, S.X.; Wang, B.; Hu, J.; Fu, X.Z.; Liu, P. Various facet tunable ZnO crystals by a scalable solvothermal synthesis and their facet-dependent photocatalytic activities. J. Phys. Chem. C 2014, 18, 25434–25440. [Google Scholar] [CrossRef]
- Imamur, S.; Ikebata, M.; Ito, T.; Ogita, T. Decomposition of ozone on a silver catalyst. Ind. Eng. Chem. Res. 1991, 30, 217–221. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, A.Q.; Ma, J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Dai, Z.; Qin, F.; Zhao, H.P.; Zhao, S.; Chen, R. Z-scheme BiO1-xBr/Bi2O2CO3 photocatalyst with rich oxygen vacancy as electron mediator for highly efficient degradation of antibiotics. Appl. Catal. B: Environ. 2017, 205, 281–291. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.H.; Lin, F.; Guo, F.S.; Alamry, K.A.; Taib, L.A.; Asiri, A.M.; Wang, X.C. Sulfur-doped carbon nitride polymers for photocatalytic degradation of organic pollutant and reduction of Cr(VI). Molecules 2017, 22, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.H.; Zhang, X.Y.; Li, G.W.; Cao, Y.T.; Shao, Y.; Li, D.Z. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B: Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Dong, Y.M.; Yang, H.X.; He, K.; Wu, X.; Zhang, A.M. Catalytic activity and stability of Y zeolite for phenol degradation in the presence of ozone. Appl. Catal. B: Environ. 2008, 82, 163–168. [Google Scholar] [CrossRef]
- Yang, T.T.; Peng, J.M.; Zheng, Y.; He, X.; Hou, Y.; Wu, L.; Fu, X. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl. Catal. B: Environ. 2018, 221, 223–234. [Google Scholar] [CrossRef]
- Li, W.J.; Li, D.Z.; Lin, Y.M.; Wang, P.X.; Chen, W.; Fu, X.Z.; Shao, Y. Evidence for the active species involved in the photodegradation process of methyl orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Lu, T.; Ming, H.; Ding, Z.; Yu, Z.; Zhang, J.; Hou, Y. Enhanced Photocatalytic Ozonation of Phenol by Ag/ZnO Nanocomposites. Catalysts 2019, 9, 1006. https://doi.org/10.3390/catal9121006
Peng J, Lu T, Ming H, Ding Z, Yu Z, Zhang J, Hou Y. Enhanced Photocatalytic Ozonation of Phenol by Ag/ZnO Nanocomposites. Catalysts. 2019; 9(12):1006. https://doi.org/10.3390/catal9121006
Chicago/Turabian StylePeng, Junmin, Tong Lu, Hongbo Ming, Zhengxin Ding, Zhiyang Yu, Jinshui Zhang, and Yidong Hou. 2019. "Enhanced Photocatalytic Ozonation of Phenol by Ag/ZnO Nanocomposites" Catalysts 9, no. 12: 1006. https://doi.org/10.3390/catal9121006