Effect of Ni–Mo Carbide Catalyst Formation on Furfural Hydrogenation
Abstract
:1. Introduction
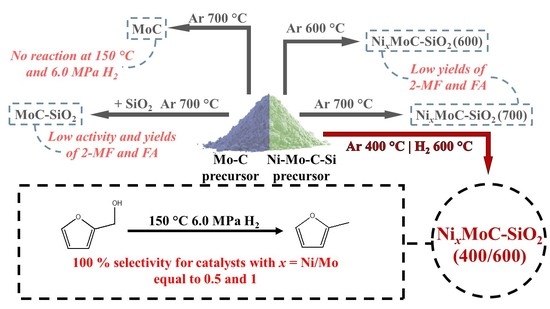
2. Results and Discussion
2.1. Catalytic Performance of Carbide Catalysts In furfural Hydrogenation Process
2.2. Phase Composition of Ni–Mo Carbide Catalysts
2.3. Texture Characterization, Active Surface and Carbon Content of Reduced Catalysts
2.4. Surface Composition of Ni–Mo Carbide Catalysts
2.5. Catalysts Characterization by Transmission Electron Microscopy
2.6. Kinetics Modeling of Furfural Hydrogenation over Reduced Ni–Mo Carbide Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. X-ray Diffraction
3.3. Texture Characteristics
3.4. CO Pulse Chemisorption Measurements
3.5. Elemental Analysis
3.6. X-ray Photoelectron Spectroscopy
3.7. Transmission Electron Microscopy
3.8. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sadaba, I.; Lopez Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Dashtban, M.; Gilbert, A.; Fatehi, P. Production of furfural: Overview and challenges. J. Sci. Technol. Forest. Prod. Process. 2012, 2, 44–53. [Google Scholar]
- Dutta, S.; De, S.; Saha, B.; Alam, M.I. Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels. Catal. Sci. Technol. 2012, 2, 2025–2036. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Barr, J.B.; Wallon, S.B. The chemistry of furfuryl alcohol resins. J. Appl. Polym. Sci. 2003, 15, 1079–1090. [Google Scholar] [CrossRef]
- Rasina, D.; Lombi, A.; Santoro, S.; Ferlin, F.; Vaccaro, L. Searching for novel reusable biomass-derived solvents: Furfuryl alcohol/water azeotrope as a medium for waste-minimised copper-catalysed azide–alkyne cycloaddition. Green Chem. 2016, 18, 6380–6386. [Google Scholar] [CrossRef]
- Kottke, R. Furan derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Bayan, S.; Beati, E. Furfural and its derivatives as motor fuels. Chim. Ind. 1941, 23, 432–434. [Google Scholar]
- Wang, C.; Xu, H.; Daniel, R.; Ghafourian, A.; Herreros, J.M.; Shuai, S.; Ma, X. Combustion characteristics and emissions of 2-methylfuran compared to 2,5-dimethylfuran, gasoline and ethanol in a DISI engine. Fuel 2013, 103, 200–211. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M. Production of high quality diesel from cellulose and hemicellulose by the Sylvan process: Catalysts and process variables. Energy Environ. Sci. 2012, 5, 6328–6344. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia Eric, R.; Bell Alexis, T. Selective Hydrogenation of Furan-Containing Condensation Products as a Source of Biomass-Derived Diesel Additives. ChemSusChem 2014, 7, 2796–2800. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2011, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, B.H. Catalytic Hydrogenation of Furan Compounds. Ind. Eng. Chem. 1948, 40, 210–216. [Google Scholar] [CrossRef]
- Burnett, L.W.; Johns, I.B.; Holdren, R.F.; Hixon, R.M. Production of 2-Methylfuran by Vapor-Phase Hydrogenation of Furfural. Ind. Eng. Chem. 1948, 40, 502–505. [Google Scholar] [CrossRef]
- Holdren, R.F. Manufacture of Methylfuran. U.S. Patent 2,445,714, 20 July 1948. [Google Scholar]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Yan, K.; Chen, A. Efficient hydrogenation of biomass-derived furfural and levulinic acid on the facilely synthesized noble-metal-free Cu–Cr catalyst. Energy 2013, 58, 357–363. [Google Scholar] [CrossRef]
- Iqbal, S.; Liu, X.; Aldosari, O.F.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Akram, A.; King, G.M.; Davies, T.E.; Morgan, D.J.; et al. Conversion of furfuryl alcohol into 2-methylfuran at room temperature using Pd/TiO2 catalyst. Catal. Sci. Technol. 2014, 4, 2280–2286. [Google Scholar] [CrossRef]
- Vetere, V.; Merlo, A.B.; Ruggera, J.F.; Casella, M.L. Transition metal-based bimetallic catalysts for the chemoselective hydrogenation of furfuraldehyde. J. Braz. Chem. Soc. 2010, 21, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K.; Liptaj, T. Effect of catalyst and solvent on the furan ring rearrangement to cyclopentanone. Appl. Catal. A Gen. 2012, 437–438, 104–111. [Google Scholar] [CrossRef]
- Patel, M.A.; Baldanza, M.A.S.; Teixeira da Silva, V.; Bridgwater, A.V. In situ catalytic upgrading of bio-oil using supported molybdenum carbide. Appl. Catal. A Gen. 2013, 458, 48–54. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, F.; Zhang, X.; Ahishakiye, R.; Xie, Y.; Shen, Y. Selective hydrodeoxygenation of guaiacol to phenolics over activated carbon supported molybdenum catalysts. Mol. Catal. 2017, 441, 28–34. [Google Scholar] [CrossRef]
- Chen, C.-J.; Lee, W.-S.; Bhan, A. Mo2C catalyzed vapor phase hydrodeoxygenation of lignin-derived phenolic compound mixtures to aromatics under ambient pressure. Appl. Catal. A Gen. 2016, 510, 42–48. [Google Scholar] [CrossRef]
- Xiong, K.; Lee, W.S.; Bhan, A.; Chen, J.G. Molybdenum carbide as a highly selective deoxygenation catalyst for converting furfural to 2-methylfuran. ChemSusChem 2014, 7, 2146–2149. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Yu, W.; Chen, J.G. Selective deoxygenation of aldehydes and alcohols on molybdenum carbide (Mo2C) surfaces. Appl. Surf. Sci. 2014, 323, 88–95. [Google Scholar] [CrossRef]
- Boullosa-Eiras, S.; Lødeng, R.; Bergem, H.; Stöcker, M.; Hannevold, L.; Blekkan, E.A. Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts. Catal. Today 2014, 223, 44–53. [Google Scholar] [CrossRef]
- Lee, W.-S.; Wang, Z.; Zheng, W.; Vlachos, D.G.; Bhan, A. Vapor phase hydrodeoxygenation of furfural to 2-methylfuran on molybdenum carbide catalysts. Catal. Sci. Technol. 2014, 4, 2340–2352. [Google Scholar] [CrossRef]
- Ma, R.; Cui, K.; Yang, L.; Ma, X.; Li, Y. Selective catalytic conversion of guaiacol to phenols over a molybdenum carbide catalyst. Chem. Commun. 2015, 51, 10299–10301. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, B.; St. Clair, T.; Oyama, S.T. Simultaneous hydrodesulfurization, hydrodeoxygenation, and hydrogenation with molybdenum carbide. Appl. Catal. A Gen. 1998, 168, 219–228. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Ling, L.-L.; Jiang, H. Selective hydrogenation of lignin to produce chemical commodities by using a biochar supported Ni–Mo2C catalyst obtained from biomass. Green Chem. 2016, 18, 4032–4041. [Google Scholar] [CrossRef]
- Hartline, A.G.; Campbell, J.A.; Magel, T.T. Process for Reclaiming Cemented Metal Carbide. U.S. Patent 3,953,194 (A), 27 April 1976. [Google Scholar]
- Smirnov, A.A.; Geng, Z.; Khromova, S.A.; Zavarukhin, S.G.; Bulavchenko, O.A.; Saraev, A.A.; Kaichev, V.V.; Ermakov, D.Y.; Yakovlev, V.A. Nickel molybdenum carbides: Synthesis, characterization, and catalytic activity in hydrodeoxygenation of anisole and ethyl caprate. J. Catal. 2017, 354, 61–77. [Google Scholar] [CrossRef]
- Khromova, S.A.; Bykova, M.V.; Bulavchenko, O.A.; Ermakov, D.Y.; Saraev, A.A.; Kaichev, V.V.; Venderbosch, R.H.; Yakovlev, V.A. Furfural Hydrogenation to Furfuryl Alcohol over Bimetallic Ni–Cu Sol–Gel Catalyst: A Model Reaction for Conversion of Oxygenates in Pyrolysis Liquids. Top. Catal. 2016, 59, 1413–1423. [Google Scholar] [CrossRef]
- Capeletti, M.A.R.; Balzano, L.; de la Puente, G.; Laborde, M.; Sedran, U. Synthesis of acetal (1,1-diethoxyethane) from ethanol and acetaldehyde over acidic catalysts. Appl. Catal. A Gen. 2000, 198, L1–L4. [Google Scholar] [CrossRef]
- Bej, S.K.; Bennett, C.A.; Thompson, L.T. Acid and base characteristics of molybdenum carbide catalysts. Appl. Catal. A Gen. 2003, 250, 197–208. [Google Scholar] [CrossRef]
- Vitale, G.; Frauwallner, M.L.; Scott, C.E.; Pereira-Almao, P. Preparation and characterization of low-temperature nano-crystalline cubic molybdenum carbides and insights on their structures. Appl. Catal. A Gen. 2011, 408, 178–186. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, K.; Jiang, D.; Li, D.; Sun, Y. Sol–gel derived Ni–Mo bimetallic carbide catalysts and their performance for CO hydrogenation. Catal. Today 2010, 158, 490–495. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Khromova, S.A.; Ermakov, D.Y.; Bulavchenko, O.A.; Saraev, A.A.; Aleksandrov, P.V.; Kaichev, V.V.; Yakovlev, V.A. The composition of Ni-Mo phases obtained by NiMoOx-SiO2 reduction and their catalytic properties in anisole hydrogenation. Appl. Catal. A Gen. 2016, 514, 224–234. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Y.; Li, Y.-W.; Jiao, H. Mechanisms of Mo2C(101)-Catalyzed Furfural Selective Hydrodeoxygenation to 2-Methylfuran from Computation. ACS Catal. 2016, 6, 6790–6803. [Google Scholar] [CrossRef]
- Rodiansono, R.; Hara, T.; Shimazu, S. Total hydrogenation of biomass-derived furfural over raney nickel-clay nanocomposite catalysts. Indones. J. Chem. 2013, 13, 101–107. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Pushkarev, V.V.; Musselwhite, N.; An, K.; Alayoglu, S.; Somorjai, G.A. High structure sensitivity of vapor-phase furfural decarbonylation/hydrogenation reaction network as a function of size and shape of Pt nanoparticles. Nano Lett. 2012, 12, 5196–5201. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Stux, A.M.; Laberty-Robert, C.; Swider-Lyons, K.E. Pechini synthesis and characterization of molybdenum carbide and nickel molybdenum carbide. J. Solid State Chem. 2008, 181, 2741–2747. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709. [Google Scholar] [CrossRef]














| Entry | Catalyst | Contact Time, Min | Furfural Conversion, mol % | Yields of Reaction Products, mol % | |||
|---|---|---|---|---|---|---|---|
| FA | 2-MF | THFA | 2-MTHF | ||||
| 1 | Ni0.5MoC–SiO2 (600) | 180 | 58 | 54 | 4 | - | - |
| 2 | Ni1MoC–SiO2 (600) | 60 | 92 | 70 | 9 | 12 | 1 |
| 3 | Ni2MoC–SiO2 (600) | 20 | 79 | 40 | 15 | 22 | 2 |
| 4 | Ni6MoC–SiO2 (600) | 40 | 66 | 34 | 7 | 23 | 2 |
| 5 | Ni0.5MoC–SiO2 (700) | 140 | 95 | 67 | 15 | 12 | 1 |
| 6 | Ni1MoC–SiO2 (700) | 80 | 85 | 56 | 16 | 11 | 2 |
| 7 | Ni2MoC–SiO2 (700) | 30 | 86 | 58 | 6 | 20 | 2 |
| 8 | Ni6MoC–SiO2 (700) | 20 | 89 | 41 | 10 | 30 | 2 |
| 9 | Ni0.5MoC–SiO2 (400/600) | 210 | 69 | 55 | 14 | - | - |
| 10 | Ni1MoC–SiO2 (400/600) | 210 | 91 | 60 | 29 | 2 | - |
| 11 | Ni2MoC–SiO2 (400/600) | 140 | 82 | 71 | 7 | 4 | - |
| 12 | Ni4MoC–SiO2 (400/600) | 140 | 95 | 76 | 5 | 14 | - |
| 13 | Ni6MoC–SiO2 (400/600) | 80 | 94 | 80 | 4 | 9 | 1 |
| Catalyst | Ni Content, wt % | Average Crystallite Size of Ni, Å | Ni Content, wt % | Average Crystallite Size of NiO, Å |
|---|---|---|---|---|
| Ni0.5MoC–SiO2 (600) | 25 | 100 | 75 | 35 |
| Ni1MoC–SiO2 (600) | 40 | 115 | 60 | 25 |
| Ni2MoC–SiO2 (600) | 40 | 130 | 60 | 27 |
| Ni6MoC–SiO2 (600) | 88 | 85 | 12 | 45 |
| Catalyst | Phase | Content of Phase, wt % | Average Crystallite Size, Å | Lattice Parameter of Ni–Mo alloy, Å |
|---|---|---|---|---|
| Ni0.5MoC–SiO2 (700) | hcp-Mo2C | 23 | 160 | |
| fcc-MoC1−x | 49 | – | ||
| Ni0.91Mo0.09 | 28 | 150 | 3.559 | |
| Ni1MoC–SiO2 (700) | hcp-Mo2C | 30 | 400 | |
| fcc-MoC1−x | 34 | – | ||
| Ni0.92Mo0.08 | 36 | 350 | 3.558 | |
| Ni2MoC–SiO2 (700) | hcp-Mo2C | 29 | 630 | |
| fcc-MoC1−x | 8 | – | ||
| Ni0.92Mo0.08 | 63 | 220 | 3.556 | |
| Ni6MoC–SiO2 (700) | hcp-Mo2C | 4 | 250 | |
| fcc-MoC1−x | 19 | – | ||
| Ni0.93Mo0.07 | 77 | 130 | 3.554 |
| Catalyst | Phase | Content of Phase, wt % | Average Crystallite Size, Å | Lattice Parameter of Ni–Mo alloy, Å |
|---|---|---|---|---|
| Ni0.5MoC–SiO2 (400/600) | hcp-Mo2C | 17 | - | 3.586 |
| fcc-MoC1−x | 51 | - | ||
| Mo3СNi2 | 4 | - | ||
| Ni0.84Mo0.16 | 28 | 70 | ||
| Ni1MoC–SiO2 (400/600) | hcp-Mo2C | 17 | 220 | 3.573 |
| fcc-MoC1−x | 0 | – | ||
| Mo3СNi2 | 41 | 350 | ||
| Ni0.88Mo0.12 | 42 | 160 | ||
| Ni2MoC–SiO2 (400/600) | hcp-Mo2C | 14 | 170 | 3.577 |
| fcc-MoC1−x | 0 | - | ||
| Mo3СNi2 | 2 | - | ||
| Ni0.87Mo0.13 | 84 | 110 | ||
| Ni4MoC–SiO2 (400/600) | hcp-Mo2C | 8 | 220 | 3.576 |
| fcc-MoC1−x | 0 | - | ||
| Mo3СNi2 | 2 | - | ||
| Ni0.87Mo0.13 | 90 | 160 | ||
| Ni6MoC–SiO2 (400/600) | hcp-Mo2C | 1 | - | 3.580 3.545 |
| fcc-MoC1−x | 0 | - | ||
| Mo3СNi2 | 3 | - | ||
| Ni0.86Mo0.14 | 65 | 160 | ||
| Ni0.95Mo0.05 | 31 | 680 |
| Catalyst | The Number of Active Sites Determined by CO Chemisorption, μmol/g |
|---|---|
| Ni0.5MoC–SiO2 | 90.4 |
| Ni1MoC–SiO2 | 64.7 |
| Ni2MoC–SiO2 | 65.5 |
| Ni4MoC–SiO2 | 8.0 |
| Ni6MoC–SiO2 | 11.9 |
| Catalyst | ABET, m2/g | V, cm3/g | D, nm |
|---|---|---|---|
| Ni0.5MoC–SiO2 (400/600) | 11.29 | 0.0149 | 5.27 |
| Ni1MoC–SiO2 (400/600) | 11.35 | 0.0189 | 6.67 |
| Ni2MoC–SiO2 (400/600) | 27.75 | 0.0307 | 4.42 |
| Ni4MoC–SiO2 (400/600) | 43.43 | 0.0512 | 4.71 |
| Ni6MoC–SiO2 (400/600) | 23.17 | 0.0574 | 9.91 |
| Catalyst | Ni/Si | Mo/Si | O/Si | C/Si | Ni/Mo |
|---|---|---|---|---|---|
| Ni1MoC–SiO2 (400/600) | 0.87 | 3.04 | 4.84 | 2.02 | 0.29 |
| Ni2MoC–SiO2 (400/600) | 0.69 | 1.08 | 2.75 | 2.24 | 0.65 |
| Ni4MoC–SiO2 (400/600) | 0.45 | 0.24 | 1.55 | 0.20 | 1.50 |
| Ni6MoC–SiO2 (400/600) | 0.77 | 0.18 | 1.54 | 0.34 | 4.56 |
| Catalyst | k0·103, min−1 | k1·103, min−1 | k2·103, min−1 | k3·103, min−1 |
|---|---|---|---|---|
| Ni0.5MoC–SiO2 | 3.4 ± 0.1 | – | 3.6 ± 0.7 | – |
| Ni1MoC–SiO2 | 5.3 ± 0.1 | – | 3.2 ± 0.5 | – |
| Ni2MoC–SiO2 | 15 ± 0.6 | 1.1 ± 0.2 | 2.6 ± 0.3 | – |
| Ni4MoC–SiO2 | 21 ± 0.5 | 1.6 ± 0.2 | 1.3 ± 0.1 | – |
| Ni6MoC–SiO2 | 39 ± 2 | 2.4 ± 0.2 | 1.3 ± 0.2 | 2.4 ± 0.2 |
| Catalyst | TOF, s−1 |
|---|---|
| Ni0.5MoC–SiO2 | 0.016 ± 0.001 |
| Ni1MoC–SiO2 | 0.035 ± 0.001 |
| Ni2MoC–SiO2 | 0.097 ± 0.004 |
| Ni4MoC–SiO2 | 1.11 ± 0.03 |
| Ni6MoC–SiO2 | 1.39 ± 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilov, I.N.; Smirnov, A.A.; Bulavchenko, O.A.; Yakovlev, V.A. Effect of Ni–Mo Carbide Catalyst Formation on Furfural Hydrogenation. Catalysts 2018, 8, 560. https://doi.org/10.3390/catal8110560
Shilov IN, Smirnov AA, Bulavchenko OA, Yakovlev VA. Effect of Ni–Mo Carbide Catalyst Formation on Furfural Hydrogenation. Catalysts. 2018; 8(11):560. https://doi.org/10.3390/catal8110560
Chicago/Turabian StyleShilov, Ivan N., Andrey A. Smirnov, Olga A. Bulavchenko, and Vadim A. Yakovlev. 2018. "Effect of Ni–Mo Carbide Catalyst Formation on Furfural Hydrogenation" Catalysts 8, no. 11: 560. https://doi.org/10.3390/catal8110560