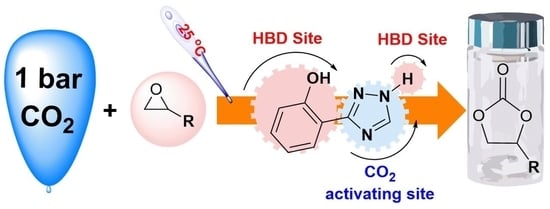
Organocatalysts for the Synthesis of Cyclic Carbonates under the Conditions of Ambient Temperature and Atmospheric CO2 Pressure
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Synthesis of Known Compounds
3.2. Synthesis of 2–(2–Benzyl–2H–tetrazol–5–yl)phenol (CAT–6)
3.3. Representative Procedure for the Coupling of Terminal Epoxide and CO2 at Ambient Condition
3.4. Representative Procedure for the Coupling of Internal Epoxide and CO2 at Ambient Condition
3.5. X-ray Crystallographic Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bobbink, F.D.; Van Muyden, A.P.; Dyson, P.J. En route to CO2-containing renewable materials: Catalytic synthesis of polycarbonates and non-isocyanate polyhydroxyurethanes derived from cyclic carbonates. Chem. Commun. 2019, 55, 1360–1373. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Weidlich, T.; Kamenická, B. Utilization of CO2-available organocatalysts for reactions with industrially important epoxides. Catalysts 2022, 12, 298. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kühn, F.E. Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. ChemSusChem 2015, 8, 2436–2454. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Fiorani, G.; Guo, W.; Kleij, A.W. Sustainable conversion of carbon dioxide: The advent of organocatalysis. Green Chem. 2015, 17, 1375–1389. [Google Scholar] [CrossRef]
- Yingcharoen, P.; Kongtes, C.; Arayachukiat, S.; Suvarnapunya, K.; Vummaleti, S.V.C.; Wannakao, S.; Cavallo, L.; Poater, A.; D’Elia, V. Assessing the pKa–dependent activity of hydroxyl hydrogen bond donors in the organocatalyzed cycloaddition of carbon dioxide to epoxides: Experimental and theoretical study. Adv. Synth. Catal. 2019, 361, 366–373. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Kongtes, C.; Barthel, A.; Vummaleti, S.V.C.; Poater, A.; Wannakao, S.; Cavallo, L.; D’Elia, V. Ascorbic acid as a bifunctional hydrogen bond donor for the Synthesis of cyclic carbonates from CO2 under ambient conditions. ACS Sustain. Chem. Eng. 2017, 5, 6392–6397. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Kodama, K.; Hirose, T. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions. Green Chem. 2016, 18, 1229–1233. [Google Scholar] [CrossRef]
- Hardman-Baldwin, A.M.; Mattson, A.E. Silanediol-catalyzed carbon dioxide fixation. ChemSusChem 2014, 7, 3275–3278. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, C.; Rodriguez, J.; Quintard, A. Organocatalytic carbon dioxide fixation to epoxides by perfluorinated 1,3,5-triols catalysts. Org. Biomol. Chem. 2020, 18, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, Y.; Kim, H.; Cho, H.J.; Baik, M.-H.; Kim, Y. Scorpionate catalysts for coupling CO2 and epoxides to cyclic carbonates: A rational design approach for organocatalysts. J. Org. Chem. 2018, 83, 9370–9380. [Google Scholar] [CrossRef]
- Liu, N.; Xie, Y.-F.; Wang, C.; Li, S.-J.; Wei, D.; Li, M.; Dai, B. Cooperative multifunctional organocatalysts for ambient conversion of carbon dioxide into cyclic carbonates. ACS Catal. 2018, 8, 9945–9957. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.-X.; Zhang, W.-Z.; Lu, X.-B. CO2 adducts of phosphorus ylides: Highly active organocatalysts for carbon dioxide transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- Wu, X.; Chen, C.; Guo, Z.; North, M.; Whitwood, A.C. Metal- and halide-free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide. ACS Catal. 2019, 9, 1895–1906. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y. Boronic acids as hydrogen bond donor catalysts for efficient conversion of CO2 into organic carbonate in water. ACS Catal. 2016, 6, 4871–4876. [Google Scholar] [CrossRef]
- Gennen, S.; Alves, M.; Mereau, R.; Tassaing, T.; Gilbert, B.; Detrembleur, C.; Jerome, C.; Grignard, B. Fluorinated alcohols as activators for the solvent–free chemical fixation of carbon dioxide into epoxides. ChemSusChem 2015, 8, 1845–1849. [Google Scholar] [CrossRef]
- Martinez–Rodriguez, L.; Garmilla, J.O.; Kleij, A.W. Cavitand-based polyphenols as highly reactive organocatalysts for the coupling of carbon dioxide and oxiranes. ChemSusChem 2016, 9, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Sopena, S.; Fiorani, G.; Martin, C.; Kleij, A.W. Highly efficient organocatalyzed conversion of oxiranes and CO2 into organic carbonates. ChemSusChem 2015, 8, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Grignard, B.; Gennen, S.; Detrembleur, C.; Jerome, C.; Tassaing, T. Organocatalytic synthesis of bio-based cyclic carbonates from CO2 and vegetable oils. RSC Adv. 2015, 5, 53629–53636. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Henseler, A.H.; Ayats, C.; Kleij, A.W.; Pericas, M.A. Conversion of oxiranes and CO2 to organic cyclic carbonates using a recyclable, bifunctional polystyrene-supported organocatalysts. Green Chem. 2014, 16, 1552–1559. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Nova, A.; Maseras, F.; Kleij, A.W. Merging sustainability with organocatalysis in the formation of organic carbonates by using CO2 as a feedstock. ChemSusChem 2012, 5, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Sun, J.; Cheng, W.-G.; Dong, K.; Zhang, X.-P.; Zhang, S.-J. Experimental and theoretical studies on hydrogen bond-promoted fixation of carbon dioxide and epoxides in cyclic carbonates. Phys. Chem. Chem. Phys. 2012, 14, 11021–11026. [Google Scholar] [CrossRef]
- Takaishi, K.; Okuyama, T.; Kadosaki, S.; Uchiyama, M.; Ema, T. Hemisquaramide tweezers as organocatalysts: Synthesis of cyclic carbonates from epoxides and CO2. Org. Lett. 2019, 21, 1397–1401. [Google Scholar] [CrossRef]
- Fan, Y.; Tiffner, M.; Schörgenhumer, J.; Robiette, R.; Waser, M.; Kass, S.R. Synthesis of cyclic organic carbonates using atmospheric pressure CO2 and charge–containing thiourea catalysts. J. Org. Chem. 2018, 83, 9991–10000. [Google Scholar] [CrossRef] [PubMed]
- Pagacz-Kostrzewa, M.; Saldyka, M.; Wierzejewska, M.; Khomenko, D.M.; Doroschuk, R.O. Theoretical DFT and matrix isolation FT IR studies of 2-(1,2,4-triazolyl)phenol isomers. Chem. Phys. Lett. 2016, 657, 156–161. [Google Scholar] [CrossRef]
- Wada, S.; Kushida, T.; Itagaki, H.; Shibue, T.; Kadowaki, H.; Arakawa, J.; Furukawa, Y. 13C NMR study on carbamate hydrolysis reactions in aqueous amine/CO2 solutions. Int. J. Greenh. Gas Control 2021, 104, 103175. [Google Scholar] [CrossRef]
- Kumar, S.; Jaller, D.; Patel, B.; LaLonde, J.M.; DuHadaway, J.B.; Malachowski, W.P.; Prendergast, G.C.; Muller, A.J. Structure based development of phenylimidazole-derived inhibitors of indoleamine 2,3-dioxygenase. J. Med. Chem. 2008, 51, 4968–4977. [Google Scholar] [CrossRef]
- Padwa, A.; Nahm, S.; Sato, E. Intramolecular 1,3-dipolar cycloaddition reactions of alkenyl-substituted nitrile imines. J. Org. Chem. 1978, 43, 1664–1671. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Martinez, J.; de la Cruz-Martinez, F.; Caballero, M.P.; Fernandez-Baeza, J.; Rodriguez-Lopez, J.; Otero, A.; Lara-Sanchez, A.; Tejeda, J. Development of hydroxy-containing imidazole organocatalysts for CO2 fixation into cyclic carbonates. Catal. Sci. Technol. 2018, 8, 1981–1987. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Escudero-Adán, E.C.; Martin, E.; Kleij, A.W. A powerful aluminum catalyst for the synthesis of highly functional organic carbonates. J. Am. Chem. Soc. 2013, 135, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Shin, M.S.; Hwang, S.; Kim, H.; Kim, M.; Kim, J.G.; Kim, Y. Tertiary amines: A new class of highly efficient organocatalysts for CO2 fixations. J. Ind. Eng. Chem. 2016, 44, 210–215. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A triazole-containing metal-organic framework as a highly effective and substrate size-dependent catalyst for CO2 conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef]
- Paddock, R.L.; Nguyen, S.T. Chemical CO2 fixation: Cr(III) salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J. Am. Chem. Soc. 2001, 123, 11498–11499. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, S.H.; Ahn, D.; Kim, Y.; Ryu, J.Y.; Lee, J.; Kim, Y. Facile synthesis of a dimeric titanium(IV) complex with terminal Ti=O moieties and its application as a catalyst for the cycloaddition reaction of CO2 to epoxides. RSC Adv. 2016, 6, 97800–97807. [Google Scholar] [CrossRef]
- Stewart, J.A.; Drexel, R.; Arstad, B.; Reubsaet, E.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Homogeneous and heterogenized masked N-heterocyclic carbenes for bio-based cyclic carbonate synthesis. Green Chem. 2016, 18, 1605–1618. [Google Scholar] [CrossRef]
- Jose, T.; Canellas, S.; Pericas, M.A.; Kleij, A.W. Polystyrene-supported bifunctional resorcinarenes as cheap, metal-free and recyclable catalysts for epoxides/CO2 coupling reactions. Green Chem. 2017, 19, 5488–5493. [Google Scholar] [CrossRef]
- Saptal, V.B.; Nanda, B.; Parida, K.M.; Bhanage, B.M. Fabrication of amine and zirconia on MCM-41 as acid-base catlysts for the fixation of carbon dioxide. ChemCatChem 2017, 9, 4105–4111. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, S.; Zhang, L.; Cheng, J.-P. Magnetic nanoparticle supported ionic liquid catalysts for CO2 cycloaddition reactions. Green Chem. 2009, 11, 455–458. [Google Scholar] [CrossRef]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and convenient procedure for solvent purification. Organometallics 1996, 15, 1518–1520. [Google Scholar] [CrossRef]
- Shelkar, R.; Singh, A.; Nagarkar, J. Amberlyst-15 catalyzed synthesis of 5-substituted 1-H-tetrazole via [3+2] cycloaddition of nitriles and sodium azide. Tetrahedron Lett. 2013, 54, 106–109. [Google Scholar] [CrossRef]





| Entry 1 | Catalyst | Conversion (%) 2 | Yield (%) 3 | TON 4 | TOF (h−1) 5 |
|---|---|---|---|---|---|
| 1 | Phenol | 0 | 0 | 0 | 0 |
| 2 | 1H–1,2,4–triazole | 24 | 19 | 4.80 | 0.40 |
| 3 | Phenol+1H–1,2,4–triazole | 26 | 23 | 5.20 | 0.43 |
| 4 | CAT–1 | 96 | 93 | 19.2 | 1.60 |
| 5 | CAT–2 | 88 | 87 | 17.6 | 1.47 |
| 6 | CAT–3 | 38 | 34 | 7.60 | 0.63 |
| 7 | CAT–4 | 3 | 1 | 0.60 | 0.05 |
| 8 | CAT–5 | 4 | 2 | 0.80 | 0.07 |
| 9 | CAT–6 | 4 | 3 | 0.80 | 0.07 |

| Entry 1 | Cocatalyst | Conversion (%) 2 | Yield (%) 3 | TON 4 | TOF (h−1) 5 |
|---|---|---|---|---|---|
| 1 | nBu4NI | 96 | 93 | 19.2 | 1.60 |
| 2 | PPNCl | 8 | 7 | 1.60 | 0.13 |
| 3 | nBu4NBr | 66 | 61 | 13.2 | 1.10 |
| 4 | nBu4NCl | 17 | 16 | 3.40 | 0.28 |
| 5 | DMAP | 0 | 0 | 0 | 0 |
| 6 | KI | 1 | 1 | 0.20 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, Y.; Lee, S.; Cho, S.; Kim, Y.; Kim, Y. Organocatalysts for the Synthesis of Cyclic Carbonates under the Conditions of Ambient Temperature and Atmospheric CO2 Pressure. Catalysts 2024, 14, 90. https://doi.org/10.3390/catal14010090
Seong Y, Lee S, Cho S, Kim Y, Kim Y. Organocatalysts for the Synthesis of Cyclic Carbonates under the Conditions of Ambient Temperature and Atmospheric CO2 Pressure. Catalysts. 2024; 14(1):90. https://doi.org/10.3390/catal14010090
Chicago/Turabian StyleSeong, Yeongju, Sanghun Lee, Seungyeon Cho, Yoseph Kim, and Youngjo Kim. 2024. "Organocatalysts for the Synthesis of Cyclic Carbonates under the Conditions of Ambient Temperature and Atmospheric CO2 Pressure" Catalysts 14, no. 1: 90. https://doi.org/10.3390/catal14010090