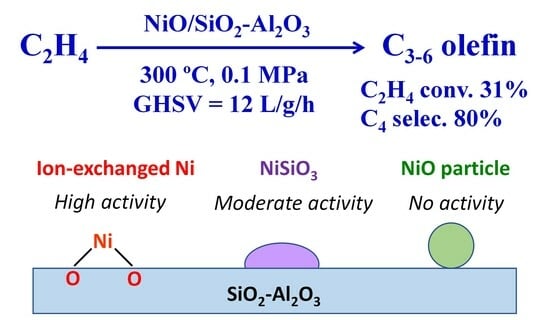
Impacts of Ni-Loading Method on the Structure and the Catalytic Activity of NiO/SiO2-Al2O3 for Ethylene Oligomerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity of Ni/ASA Prepared Using Different Ni-Loading Methods
2.2. Characterization of the Ni/ASA Catalysts and the Types of Active Ni Species
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Characterization Techniques
3.3. Activity Test for Ethylene Oligomerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finiels, A.; Fajula, F.; Hulea, V. Nickel-Based Solid Catalysts for Ethylene Oligomerization—A Review. Catal. Sci. Technol. 2014, 4, 2412–2426. [Google Scholar] [CrossRef]
- Joshi, R.; Saxena, A.; Gounder, R. Mechanistic Insights into Alkene Chain Growth Reactions Catalyzed by Nickel Active Sites on Ordered Microporous and Mesoporous Supports. Catal. Sci. Technol. 2020, 10, 7101–7123. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Breuil, P.A.R.; Magna, L.; Michel, T.; Pastor, M.F.E.; Delcroix, D. Nickel Catalyzed Olefin Oligomerization and Dimerization. Chem. Rev. 2020, 120, 7919–7983. [Google Scholar] [CrossRef]
- Betz, M.; Fuchs, C.; Zevaco, T.A.; Arnold, U.; Sauer, J. Production of Hydrocarbon Fuels by Heterogeneously Catalyzed Oligomerization of Ethylene: Tuning of the Product Distribution. Biomass Bioenergy 2022, 166, 106595. [Google Scholar] [CrossRef]
- Seufitelli, G.V.S.; Gustafson, R. Novel Ni-SIRAL Catalyst for Heterogeneous Ethylene Oligomerization. Ind. Eng. Chem. Res. 2022, 61, 4286–4299. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Abed, O.; Zambrano, N.; Castaño, P.; Hita, I. A Zeolite-Based Cascade System to Produce Jet Fuel from Ethylene Oligomerization. Ind. Eng. Chem. Res. 2022, 61, 15880–15892. [Google Scholar] [CrossRef]
- McGuinness, D.S. Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef]
- Breuil, P.R.; Magna, L.; Olivier-Bourbigou, H. Role of Homogeneous Catalysis in Oligomerization of Olefins: Focus on Selected Examples Based on Group 4 to Group 10 Transition Metal Complexes. Catal. Lett. 2015, 145, 173–192. [Google Scholar] [CrossRef]
- Heveling, J.; Beek, A.V.D.; Pender, M.D. Oligomerization of Ethene over Nickel-Exchanged Zeolite Y into a Diesel-Range Product. Appl. Catal. 1988, 42, 325–336. [Google Scholar] [CrossRef]
- Ng, F.T.T.; Creaser, D.C. Ethylene Dimerization: Kinetics and Selectivity for 1-Butene. Stud. Surf. Sci. Catal. 1992, 73, 123–131. [Google Scholar]
- Martínez, A.; Arribas, M.A.; Concepción, P.; Moussa, S. New Bifunctional Ni–H-Beta Catalysts for the Heterogeneous Oligomerization of Ethylene. Appl. Catal. A: Gen. 2013, 467, 509–518. [Google Scholar] [CrossRef]
- Moussa, S.; Concepción, P.; Arribas, M.A.; Martínez, A. Nature of Active Nickel Sites and Initiation Mechanism for Ethylene Oligomerization on Heterogeneous Ni-Beta Catalysts. ACS Catal. 2018, 8, 3903–3912. [Google Scholar] [CrossRef]
- Lee, K.; Hong, S.B. Ethene Dimerization over Ni-Beta Catalysts Prepared by Solid-State Ion exchange. Appl. Catal. A: Gen. 2021, 615, 118059. [Google Scholar] [CrossRef]
- McCaig, J.; Lamb, H.H. Ni-H-Beta Catalysts for Ethylene Oligomerization: Impact of Parent Cation on Ni Loading, Speciation, and Siting. Catalysts 2022, 12, 824. [Google Scholar] [CrossRef]
- Yamamura, M.; Chaki, K.; Wakatsuki, T.; Okado, H.; Fujimoto, K. Synthesis of ZSM-5 Zeolite with Small Crystal Size and Its Catalytic Performance for Ethylene Oligomerization. Zeolites 1994, 14, 643–649. [Google Scholar] [CrossRef]
- Ganjkhanlou, Y.; Berlier, G.; Groppo, E.; Borfecchia, E.; Bordiga, S. In Situ Investigation of the Deactivation Mechanism in Ni-ZSM5 During Ethylene Oligomerization. Top Catal. 2017, 60, 1664–1672. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. Ni-MCM-36 and Ni-MCM-22 Catalysts for the Ethylene Oligomerization. Stud. Surf. Sci. Catal. 2008, 174, 1139–1142. [Google Scholar]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. NiMCM-36 and NiMCM-22 Catalysts for the Ethylene Oligomerization: Effect of Zeolite Texture and Nickel Cations/Acid Sites Ratio. Appl. Catal. A Gen. 2008, 338, 37–43. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Snel, R.; Korf, C.J.; Nicolaides, C.P. Catalytic Oligomerization of Ethene over Nickel-Exchanged Amorphous Silica-Aluminas; Effect of the Acid Strength of the Support. Appl. Catal. 1987, 29, 295–303. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Nicolaides, C.P.; Korf, C.J.; Snel, R. Catalytic Oligomerization of Ethene over Nickel-Exchanged Amorphous Silica-Alumina; Effect of the Nickel Concentration. Appl. Catal. 1987, 31, 259–266. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Korf, C.J.; Nicolaides, C.P.; Snel, R. Catalytic Oligomerization of Ethene over Nickel-Exchanged Amorphous Silica-Alumina; Effect of the Reaction Conditions and Modelling of the Reaction. Appl. Catal. 1987, 29, 175–184. [Google Scholar] [CrossRef]
- Heveling, J.; Nicolaides, C.P.; Scurrell, M.S. Catalysts and Conditions for the Highly Efficient, Selective and Stable Heterogeneous Oligomerisation of Ethylene. Appl. Catal. A Gen. 1998, 173, 1–9. [Google Scholar] [CrossRef]
- Heydenrych, M.D.; Nicolaides, C.P.; Scurrell, M.S. Oligomerization of Ethene in a Slurry Reactor Using a Nickel(II)-Exchanged Silica–Alumina Catalyst. J. Catal. 2001, 197, 49–57. [Google Scholar] [CrossRef]
- Toch, K.; Thybaut, J.W.; Marin, G.B. Ethene Oligomerization on Ni-SiO2-Al2O3: Experimental Investigation and Single-Event MicroKinetic Modeling. Appl. Catal. A Gen. 2015, 489, 292–304. [Google Scholar] [CrossRef]
- Yoon, J.S.; Park, M.B.; Kim, Y.; Hwang, D.W.; Chae, H. Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts. Catalysts 2019, 9, 933. [Google Scholar] [CrossRef]
- Khudhair, A.A.; Bouchmella, K.; Andrei, R.D.; Mehdi, A.; Mutin, P.H.; Hulea, V. One-Step Non-Hydrolytic Sol-Gel Synthesis of Mesoporous SiO2-Al2O3-NiO Catalysts for Ethylene Oligomerization. Microporous Mesoporous Mater. 2021, 322, 111165. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Zhang, Y.; Li, L.; Yan, W.; Wang, J.; Liu, G.; Su, X.; Huang, Y.; Zhang, T. Identification of the Structure of Ni Active Sites for Ethylene Oligomerization on an Amorphous Silica-Alumina Supported Nickel Catalyst. Chin. J. Catal. 2021, 42, 2181–2188. [Google Scholar] [CrossRef]
- Aid, A.; Andrei, R.D.; Amokrane, S.; Cammarano, C.; Nibou, D.; Hulea, V. Ni-Exchanged Cationic Clays as Novel Heterogeneous Catalysts for Selective Ethylene Oligomerization. Appl. Clay Sci. 2017, 146, 432–438. [Google Scholar] [CrossRef]
- Hulea, V.; Fajula, F. Ni-Exchanged AlMCM-41—An Efficient Bifunctional Catalyst for Ethylene Oligomerization. J. Catal. 2004, 225, 213–222. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Iglesia, E. Stabilization of Active, Selective, and Regenerable Ni-Based Dimerization Catalysts by Condensation of Ethene within Ordered Mesopores. J. Catal. 2017, 352, 505–514. [Google Scholar] [CrossRef]
- Moussa, S.; Concepción, P.; Arribas, M.A.; Martínez, A. The Nature of Active Ni Sites and the Role of Al Species in the Oligomerization of Ethylene on Mesoporous Ni-Al-MCM-41 Catalysts. Appl. Catal. A Gen. 2020, 608, 117831. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Li, W.; Ge, L.; Yu, G.; Qiu, M.; Chen, X. Tuning the Ni Site Location of Bifunctional Ni-Based Catalysts for Improving the Performance in Ethylene Oligomerization. New J. Chem. 2022, 46, 9461–9469. [Google Scholar] [CrossRef]
- Andrei, R.D.; Mureseanu, M.; Popa, M.I.; Cammarano, C.; Fajula, F.; Hulea, V. Ni-Exchanged AlSBA-15 Mesoporous Materials as Outstanding Catalysts for Ethylene Oligomerization. Eur. Phys. J. Special Topics 2015, 224, 1831–1841. [Google Scholar] [CrossRef]
- Andrei, R.D.; Popa, M.I.; Fajula, F.; Hulea, V. Heterogeneous Oligomerization of Ethylene over Highly Active and Stable Ni-AlSBA-15 Mesoporous Catalysts. J. Catal. 2015, 323, 76–84. [Google Scholar] [CrossRef]
- Andrei, R.D.; Popa, M.I.; Cammaranoa, C.; Hulea, V. Nickel and Molybdenum Containing Mesoporous Catalysts for Ethylene Oligomerization and Metathesis. New J. Chem. 2016, 40, 4146–4152. [Google Scholar] [CrossRef]
- Jan, O.; Song, K.; Dichiara, A.; Resende, F.L.P. Oligomerization of Supercritical Ethylene over Nickel-Based Silica-Alumina Catalysts. Chem. Eng. Sci. 2019, 197, 212–222. [Google Scholar] [CrossRef]
- Beucher, R.; Cammarano, C.; Rodríguez-Castellón, E.; Hulea, V. Direct Conversion of Ethylene to Propylene over Ni- and W-Based Catalysts: An Unprecedented Behaviour. Catal. Commun. 2020, 144, 106091. [Google Scholar] [CrossRef]
- Beucher, R.; Hulea, V.; Cammarano, C. Kinetic and Mechanistic Insights into Ni-AlKIT-6 Catalyzed Ethylene Oligomerization. React. Chem. Eng. 2022, 7, 133–141. [Google Scholar] [CrossRef]
- Cai, T.; Cao, D.; Song, Z.; Li, L. Catalytic Behavior of NiSO4/γ-Al2O3 for Ethene Dimerization. Appl. Catal. A Gen. 1993, 95, Ll–L7. [Google Scholar] [CrossRef]
- Zhang, Q.; Dalla Lana, I.G. An Analysis of Mass Transfer and Kinetics during Ethylene Oligomerization over Nickel/Sulfated Alumina Catalyst in a Slurry Reactor. Chem. Eng. Sci. 1997, 52, 4187–4195. [Google Scholar] [CrossRef]
- Cai, T. Studies of a New Alkene Oligomerization Catalyst Derived from Nickel Sulfate. Catal. Today 1999, 51, 153–160. [Google Scholar] [CrossRef]
- Sohn, J.R.; Park, W.C.; Kim, H.W. Characterization of Nickel Sulfate Supported on γ-Al2O3 for Ethylene Dimerization and Its Relationship to Acidic Properties. J. Catal. 2002, 209, 69–74. [Google Scholar] [CrossRef]
- Sohn, J.R.; Park, W.C.; Shin, D.C. Characterization of Nickel Sulfate Supported on SiO2 for Ethylene Dimerization and Promoting Effect of Al2O3 on Catalytic Activity. J. Mol. Catal. A Chem. 2006, 256, 156–163. [Google Scholar] [CrossRef]
- Sohn, J.R.; Lee, S.H. Effect of TiO2–ZrO2 Composition on Catalytic Activity of Supported NiSO4 for Ethylene Dimerization. Appl. Catal. A Gen. 2007, 321, 27–34. [Google Scholar] [CrossRef]
- Shin, M.; Suh, Y. Ethylene Oligomerization over SiO2–Al2O3 Supported Ni2P Catalyst. ChemCatChem 2020, 12, 135–140. [Google Scholar] [CrossRef]
- Shin, M.; Jeong, H.; Park, M.; Suh, Y. Benefits of the SiO2-Supported Nickel Phosphide Catalyst on Ethylene Oligomerization. Appl. Catal. A Gen. 2020, 591, 117376. [Google Scholar] [CrossRef]
- Henry, R.; Komurcu, M.; Ganjkhanlou, Y.; Brogaard, R.Y.; Lu, L.; Jens, K.; Berlier, G.; Olsbye, U. Ethene Oligomerization on Nickel Microporous and Mesoporous-Supported Catalysts: Investigation of the Active Sites. Catal. Today 2018, 299, 154–163. [Google Scholar] [CrossRef]
- Moussa, S.; Arribas, M.A.; Concepción, P.; Martínez, A. Heterogeneous Oligomerization of Ethylene to Liquids on Bifunctional Ni-Based Catalysts: The Influence of Support Properties on Nickel Speciation and Catalytic Performance. Catal. Today 2016, 277, 78–88. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Kømurcu, M.; Dyballa, M.M.; Botan, A.; Speybroeck, V.V.; Olsbye, U.; Wispelaere, K.D. Ethene Dimerization on Zeolite-Hosted Ni Ions: Reversible Mobilization of the Active Site. ACS Catal. 2019, 9, 5645–5650. [Google Scholar] [CrossRef]
- Choo, H.; Kevan, L. Catalytic Study of Ethylene Dimerization on Ni(II)-Exchanged Clinoptilolite. J. Phys. Chem. B 2001, 105, 6353–6360. [Google Scholar] [CrossRef]
- Mlinar, A.N.; Shylesh, S.; Ho, O.C.; Bell, A.T. Propene Oligomerization using Alkali Metal- and Nickel-Exchanged Mesoporous Aluminosilicate Catalysts. ACS Catal. 2014, 4, 337–343. [Google Scholar] [CrossRef]
- Mlinar, A.N.; Baur, G.B.; Bong, G.G.; Getsoian, A.; Bell, A.T. Propene Oligomerization over Ni-exchanged Na-X Zeolites. J. Catal. 2012, 296, 156–164. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, S.; Oikawa, H.; Fujitani, T. Ethylene Oligomerization over NiOx/SiO2-Al2O3 Catalysts Prepared by a Coprecipitation Method. Mol. Catal. 2022, 528, 112478. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, S.; Oikawa, H.; Fujitani, T. Preparation of NiOx/SiO2-Al2O3 Catalysts by a Homogenous Precipitation Method and Their Catalytic Activity for Ethylene Oligomerization. Microporous Mesoporous Mater. 2022, 338, 111955. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, L. Hierarchical Core–Shell Structured Fe3O4@NiSiO3 Magnetic Microspheres: Preparation, Characterization, and Adsorption of Methylene Blue from Aqueous Solution. RSC Adv. 2016, 6, 49769–49776. [Google Scholar] [CrossRef]
- Joshi, R.; Zhang, G.; Miller, J.T.; Gounder, R. Evidence for the Coordination−Insertion Mechanism of Ethene Dimerization at Nickel Cations Exchanged onto Beta Molecular Sieves. ACS Catal. 2018, 8, 11407–11422. [Google Scholar] [CrossRef]
- Hulea, V. Toward Platform Chemicals from Bio-Based Ethylene: Heterogeneous Catalysts and Processes. ACS Catal. 2018, 8, 3263–3279. [Google Scholar] [CrossRef]
- Heveling, J.; Nicolaides, C.P. Chain-Length Distributions Obtained over Nickel(II)-Exchanged or Impregnated Silica–Alumina Catalysts for the Oligomerization of Lower Alkenes. Catal. Lett. 2006, 107, 117–121. [Google Scholar] [CrossRef]









| Entry | Catalyst | Conv. | Yield b (%) | Selectivity b (%) | Iso-C4H8/n-C4H8 c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C2H4 (%) | C3 | C4 | C5 | C6 | C3 | C4 | C5 | C6 | |||
| 1 | ASA | 0.58 | 0.00 | 0.10 | 0.00 | 0.03 | 0.0 | 18.0 | 0.0 | 5.7 | 0.67 |
| 2 | Ni(0.1) Imp | 9.73 | 0.00 | 8.21 | 0.22 | 0.70 | 0.0 | 84.4 | 2.3 | 7.2 | 0.30 |
| 3 | Ni(0.3) Imp | 18.14 | 0.37 | 15.06 | 0.42 | 1.48 | 2.1 | 83.0 | 2.3 | 8.2 | 0.28 |
| 4 | Ni(0.5) Imp | 27.12 | 0.66 | 21.06 | 0.52 | 2.01 | 2.4 | 77.6 | 1.9 | 7.4 | 0.21 |
| 5 | Ni(1) Imp | 26.30 | 0.64 | 20.95 | 0.51 | 1.99 | 2.4 | 79.7 | 1.9 | 7.6 | 0.21 |
| 6 | Ni(2) Imp | 26.43 | 0.66 | 20.64 | 0.53 | 2.04 | 2.5 | 78.1 | 2.0 | 7.7 | 0.21 |
| 7 | Ni(4) Imp | 24.66 | 0.63 | 19.79 | 0.48 | 1.88 | 2.5 | 80.3 | 1.9 | 7.6 | 0.21 |
| 8 | Ni(6) Imp | 26.79 | 0.56 | 21.27 | 0.45 | 1.97 | 2.1 | 79.4 | 1.7 | 7.4 | 0.21 |
| 9 | Ni(8) Imp | 22.70 | 0.49 | 19.20 | 0.42 | 1.78 | 2.2 | 84.6 | 1.8 | 7.8 | 0.21 |
| 10 | Ni(1) HP | 14.74 | 0.22 | 11.23 | 0.31 | 1.08 | 1.5 | 76.2 | 2.1 | 7.3 | 0.28 |
| 11 | Ni(2) HP | 13.79 | 0.24 | 11.09 | 0.32 | 1.01 | 1.8 | 80.4 | 2.3 | 7.3 | 0.29 |
| 12 | Ni(4) HP | 18.53 | 0.46 | 15.14 | 0.47 | 1.54 | 2.5 | 81.7 | 2.5 | 8.3 | 0.27 |
| 13 | Ni(6) HP | 24.49 | 0.52 | 19.46 | 0.49 | 1.85 | 2.1 | 79.5 | 2.0 | 7.5 | 0.21 |
| 14 | Ni(8) HP | 24.52 | 0.64 | 19.42 | 0.55 | 1.91 | 2.6 | 79.2 | 2.2 | 7.8 | 0.21 |
| 15 | Ni(10) HP | 24.38 | 0.57 | 18.96 | 0.47 | 1.79 | 2.4 | 77.8 | 1.9 | 7.3 | 0.21 |
| 16 | Ni(12) HP | 23.30 | 0.60 | 19.28 | 0.47 | 1.86 | 2.6 | 82.8 | 2.0 | 8.0 | 0.21 |
| 17 | Ni(16) HP | 18.10 | 0.44 | 14.69 | 0.35 | 1.47 | 2.4 | 81.2 | 1.9 | 8.1 | 0.26 |
| 18 | Ni(20) HP | 16.17 | 0.39 | 13.35 | 0.32 | 1.35 | 2.4 | 82.5 | 2.0 | 8.4 | 0.26 |
| 19 | Ni(1.1) IE | 31.14 | 0.80 | 24.76 | 0.57 | 2.39 | 2.6 | 79.5 | 1.8 | 7.7 | 0.21 |
| 20 | NiSiO3 | 2.01 | 0.00 | 1.87 | 0.00 | 0.06 | 0.0 | 93.0 | 0.1 | 3.0 | 0.31 |
| Entry | Catalysts | BET Specific Surface Area (m2/g) | Pore Volume (cm³/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| 1 | ASA | 757 | 0.65 | 3.45 |
| 2 | Ni(1.1) IE | 742 | 0.66 | 3.58 |
| 3 | Ni(0.1) Imp | 697 | 0.61 | 3.51 |
| 4 | Ni(0.3) Imp | 718 | 0.63 | 3.49 |
| 5 | Ni(0.5) Imp | 727 | 0.64 | 3.53 |
| 6 | Ni(1) Imp | 734 | 0.65 | 3.51 |
| 7 | Ni(2) Imp | 669 | 0.60 | 3.56 |
| 8 | Ni(4) Imp | 648 | 0.58 | 3.55 |
| 9 | Ni(6) Imp | 648 | 0.57 | 3.53 |
| 10 | Ni(8) Imp | 649 | 0.56 | 3.44 |
| 11 | Ni(1) HP | 794 | 0.68 | 3.41 |
| 12 | Ni(2) HP | 709 | 0.66 | 3.69 |
| 13 | Ni(4) HP | 703 | 0.63 | 3.61 |
| 14 | Ni(6) HP | 750 | 0.69 | 3.68 |
| 15 | Ni(8) HP | 677 | 0.61 | 3.60 |
| 16 | Ni(10) HP | 670 | 0.65 | 3.90 |
| 17 | Ni(12) HP | 665 | 0.59 | 3.54 |
| 18 | Ni(16) HP | 688 | 0.66 | 3.84 |
| 19 | Ni(20) HP | 663 | 0.59 | 3.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimura, K.; Yoshida, S.; Oikawa, H.; Fujitani, T. Impacts of Ni-Loading Method on the Structure and the Catalytic Activity of NiO/SiO2-Al2O3 for Ethylene Oligomerization. Catalysts 2023, 13, 1303. https://doi.org/10.3390/catal13091303
Shimura K, Yoshida S, Oikawa H, Fujitani T. Impacts of Ni-Loading Method on the Structure and the Catalytic Activity of NiO/SiO2-Al2O3 for Ethylene Oligomerization. Catalysts. 2023; 13(9):1303. https://doi.org/10.3390/catal13091303
Chicago/Turabian StyleShimura, Katsuya, Shigehiro Yoshida, Hiroshi Oikawa, and Tadahiro Fujitani. 2023. "Impacts of Ni-Loading Method on the Structure and the Catalytic Activity of NiO/SiO2-Al2O3 for Ethylene Oligomerization" Catalysts 13, no. 9: 1303. https://doi.org/10.3390/catal13091303