Catalytic Pyrolysis of Hydrochar by Calcined Eggshells for Bioenergy Production: Improved Thermo-Kinetic Studies and Reduced Pollutant Emissions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Hydrochar
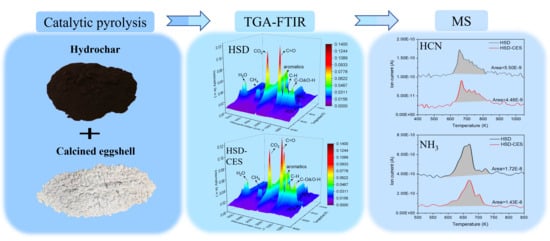
2.2. Effect of Catalyst on Thermal Decomposition Behavior of Hydrochar
2.3. Kinetic Analysis
2.4. Thermodynamics Analysis
2.5. Effect of Catalyst on Gas Evolution
3. Experimental Design
3.1. Hydrochar Preparation
3.2. Catalyst Preparation
3.3. Characterization
3.4. TG-FTIR-MS Analysis
3.5. Kinetic and Thermodynamic Analysis
3.5.1. Model-Free Methods
3.5.2. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. Thermal behavior and kinetics of co-pyrolysis of cellulose and polyethylene with the addition of transition metals. Energy Convers. Manag. 2018, 172, 32–38. [Google Scholar] [CrossRef]
- Jara-Cobos, L.; Abril-González, M.; Pinos-Vélez, V. Production of Hydrogen from Lignocellulosic Biomass: A Review of Technologies. Catalysts 2023, 13, 766. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.-H.; Zhang, Y.; Ho, S.-H. Influence of microorganisms on the variation of raw and oxidatively torrefied microalgal biomass properties. Energy 2023, 276, 127612. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, X.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst. Catalysts 2022, 12, 256. [Google Scholar] [CrossRef]
- Xu, T.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Calcined Dolomite. Catalysts 2022, 12, 131. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Carbonization temperature and feedstock type interactively affect chemical, fuel, and surface properties of hydrochars. Bioresour. Technol. 2021, 330, 124976. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Li, T.; Lei, M.; Yan, W.; Ge, S. Pyrolysis behavior of hydrochar from hydrothermal carbonization of pinewood sawdust. J. Anal. Appl. Pyrolysis 2020, 146, 104771. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Nawaz, A.; Kumar, P. Impact of temperature severity on hydrothermal carbonization: Fuel properties, kinetic and thermodynamic parameters. Fuel 2023, 336, 127166. [Google Scholar] [CrossRef]
- Iminabo, M.; Yip, A.C.K.; Iminabo, J.T.; Pang, S. Application of MgO-Titanomagnetite mixture in high-temperature catalytic pyrolysis of radiata pine. Biomass Convers. Bior. 2023, in press. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Gao, Z.; Guo, Y.; Zhang, H.; Ma, D. The Nature of Interfacial Catalysis over Pt/NiAl2O4 for Hydrogen Production from Methanol Reforming Reaction. J. Am. Chem. Soc. 2023, 145, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, M.; Jia, Z.; Li, C.; Liu, H.; Zhang, H.; Ma, D. Kinetic Evidence of Most Abundant Surface Intermediates Variation over Ptn and Ptp: Few-Atom Pt Ensembles Enable Efficient Catalytic Cyclohexane Dehydrogenation for Hydrogen Production-II. ACS Catal. 2022, 12, 7248–7261. [Google Scholar] [CrossRef]
- Yap, T.L.; Loy, A.C.M.; Chin, B.L.F.; Lim, J.Y.; Alhamzi, H.; Chai, Y.H.; Yiin, C.L.; Cheah, K.W.; Wee, M.X.J.; Lam, M.K.; et al. Synergistic effects of catalytic co-pyrolysis Chlorella vulgaris and polyethylene mixtures using artificial neuron network: Thermodynamic and empirical kinetic analyses. J. Environ. Chem. Eng. 2022, 10, 107391. [Google Scholar] [CrossRef]
- Yang, H.; Ji, G.; Clough, P.T.; Xu, X.; Zhao, M. Kinetics of catalytic biomass pyrolysis using Ni-based functional materials. Fuel Process. Technol. 2019, 195, 106145. [Google Scholar] [CrossRef]
- Gao, N.; Salisu, J.; Quan, C.; Williams, P. Modified nickel-based catalysts for improved steam reforming of biomass tar: A critical review. Renew. Sustain. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Photiou, P.; Vyrides, I. Calcined eggshells in anaerobic digestion: Buffering acidification in AD and evaluating end products from phosphate adsorption as soil conditioners. J. Environ. Chem. Eng. 2022, 10, 107957. [Google Scholar] [CrossRef]
- Raheem, A.; Liu, H.; Ji, G.; Zhao, M. Gasification of lipid-extracted microalgae biomass promoted by waste eggshell as CaO catalyst. Algal Res. 2019, 42, 101601. [Google Scholar] [CrossRef]
- Gan, D.K.W.; Loy, A.C.M.; Chin, B.L.F.; Yusup, S.; Unrean, P.; Rianawati, E.; Acda, M.N. Kinetics and thermodynamic analysis in one-pot pyrolysis of rice hull using renewable calcium oxide-based catalysts. Bioresour. Technol. 2018, 265, 180–190. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhou, N.; Dai, L.; Deng, W.; Liu, C.; Cheng, Y.; Liu, Y.; Cobb, K.; Chen, P.; et al. Applications of calcium oxide–based catalysts in biomass pyrolysis/gasification—A review. J. Clean. Prod. 2021, 291, 125826. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Nzihou, A.; Eskicioglu, C. Hydrochar derived from municipal sludge through hydrothermal processing: A critical review on its formation, characterization, and valorization. Water Res. 2021, 199, 117186. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Baloch, H.A.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A. Upgradation of chemical, fuel, thermal, and structural properties of rice husk through microwave-assisted hydrothermal carbonization. Environ. Sci. Pollut. Res. Int. 2018, 25, 17529–17539. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Xu, C.; Chen, S.; Soomro, A.; Sun, Z.; Xiang, W. Hydrogen-rich syngas production from biomass gasification using synthesized Fe/CaO active catalysts. J. Energy Inst. 2018, 91, 805–816. [Google Scholar] [CrossRef]
- Koido, K.; Kurosawa, K.; Endo, K.; Sato, M. Catalytic and inhibitory roles of K and Ca in the pyrolysis and CO2 or steam gasification of Erianthus, and their effects on co-gasification performance. Biomass Bioenergy 2021, 154, 106257. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.-H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; Hamid, M.K.A.; Isha, R.; Hasbullah, H.; Ali, R.R. Thermogravimetric catalytic pyrolysis and kinetic studies of coconut copra and rice husk for possible maximum production of pyrolysis oil. J. Clean. Prod. 2017, 167, 218–228. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Gan, D.K.W.; Yusup, S.; Chin, B.L.F.; Lam, M.K.; Shahbaz, M.; Unrean, P.; Acda, M.N.; Rianawati, E. Thermogravimetric kinetic modeling of in-situ catalytic pyrolytic conversion of rice husk to bioenergy using rice hull ash catalyst. Bioresour. Technol. 2018, 261, 213–222. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Liu, C.G.; Tawab, A.; Bai, F.W.; Sakdaronnarong, C.; Xu, J.; Rahimuddin, S.A.; Gull, M. Bioenergy potential of Wolffia arrhiza appraised through pyrolysis, kinetics, thermodynamics parameters and TG-FTIR-MS study of the evolved gases. Bioresour. Technol. 2018, 253, 297–303. [Google Scholar] [CrossRef]
- Kai, X.; Li, R.; Yang, T.; Shen, S.; Ji, Q.; Zhang, T. Study on the co-pyrolysis of rice straw and high-density polyethylene blends using TG-FTIR-MS. Energy Convers. Manag. 2017, 146, 20–33. [Google Scholar] [CrossRef]
- Ni, Z.; Bi, H.; Jiang, C.; Sun, H.; Zhou, W.; Qiu, Z.; Lin, Q. Research on the co-pyrolysis of coal slime and cellulose-based on TG-FTIR-MS, artificial neural network, and principal component analysis. Fuel 2022, 320, 123960. [Google Scholar] [CrossRef]
- Chang, G.; Miao, P.; Yan, X.; Wang, G.; Guo, Q. Phenol preparation from catalytic pyrolysis of palm kernel shell at low temperatures. Bioresour. Technol. 2018, 253, 214–219. [Google Scholar] [CrossRef]
- Burra, K.R.G.; Liu, X.; Wang, Z.; Li, J.; Che, D.; Gupta, A.K. Quantifying the sources of synergistic effects in co-pyrolysis of pinewood and polystyrene. Appl. Energy 2021, 302, 117562. [Google Scholar] [CrossRef]
- Nan, H.; Xiao, Z.; Zhao, L.; Yang, F.; Xu, H.; Xu, X.; Qiu, H. Nitrogen Transformation during Pyrolysis of Various N-Containing Biowastes with Participation of Mineral Calcium. ACS Sustain. Chem. Eng. 2020, 8, 12197–12207. [Google Scholar] [CrossRef]
- GB/T28731-2012; Methods for industrial analysis of solid biomass fuels. National Coal Standardization Technical Committee: Beijing, China, 2012.
- Lang, Q.; Zhang, B.; Liu, Z.; Chen, Z.; Xia, Y.; Li, D.; Ma, J.; Gai, C. Co-hydrothermal carbonization of corn stalk and swine manure: Combustion behavior of hydrochar by thermogravimetric analysis. Bioresour. Technol. 2019, 271, 75–83. [Google Scholar] [CrossRef] [PubMed]






| Samples | Proximate Analysis a (wt.%) | Ultimate Analysis a (wt.%) | HHV (MJ kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FC b | VM c | Ash | C | H | O d | N | S | ||
| SD | 14.31 | 84.81 | 0.90 | 46.26 | 6.37 | 46.08 | 0.46 | 0.03 | 18.86 |
| HSD | 26.52 | 72.04 | 1.52 | 54.03 | 6.10 | 38.70 | 0.85 | 0.06 | 21.65 |
| Heating Rate (K min−1) | HSD | HSD-CaO | HSD-CES | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tm a (K) | -Rp b (% min−1) | WR c (%) | Tm a (K) | -Rp b (%min−1) | WR d (%) | Tm a (K) | -Rp b (% min−1) | WR d (%) | |
| 10 | 636.05 | 9.16 | 31.50 | 634.65 | 6.16 | 29.31 | 639.35 | 6.88 | 24.59 |
| 20 | 649.55 | 17.85 | 29.33 | 649.95 | 12.01 | 27.11 | 653.15 | 13.25 | 22.63 |
| 30 | 656.55 | 26.53 | 31.13 | 658.65 | 18.56 | 27.70 | 659.85 | 19.09 | 23.81 |
| 40 | 662.25 | 23.19 | 30.04 | 664.35 | 23.53 | 29.81 | 663.65 | 24.46 | 24.53 |
| FWO | KAS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
(KJ·mol−1) | (s−1) | (KJ·mol−1) | (KJ·mol−1) | (KJ·mol−1) | (s−1) | (KJ·mol−1) | (KJ·mol−1) | ||||
| HSD | 0.2 | 196.04 | 0.969 | 6.51 × 1015 | 190.64 | 153.37 | 196.07 | 0.966 | 6.55 × 1015 | 190.67 | 153.34 |
| 0.3 | 203.11 | 0.988 | 2.50 × 1016 | 197.71 | 146.11 | 203.22 | 0.987 | 2.55 × 1016 | 197.82 | 146.00 | |
| 0.4 | 202.02 | 0.994 | 2.03 × 1016 | 196.63 | 147.22 | 201.91 | 0.993 | 1.99 × 1016 | 196.51 | 147.35 | |
| 0.5 | 196.34 | 0.998 | 6.90 × 1015 | 190.94 | 153.05 | 195.80 | 0.998 | 6.22 × 1015 | 190.40 | 153.62 | |
| 0.6 | 200.10 | 0.999 | 1.41 × 1016 | 194.70 | 149.20 | 199.62 | 0.999 | 1.29 × 1016 | 194.22 | 149.69 | |
| 0.7 | 230.78 | 0.996 | 4.77 × 1018 | 225.38 | 117.78 | 231.71 | 0.996 | 5.69 × 1018 | 226.31 | 116.83 | |
| Average | 204.73 | 9.67 × 1017 | 199.33 | 144.46 | 204.72 | 9.60 × 1017 | 199.32 | 144.47 | |||
| HSD-CES | 0.2 | 171.73 | 0.967 | 6.20 × 1013 | 166.33 | 178.59 | 170.43 | 0.963 | 4.84 × 1013 | 165.03 | 179.93 |
| 0.3 | 177.07 | 0.991 | 1.72 × 1014 | 171.67 | 173.09 | 175.76 | 0.990 | 1.34 × 1014 | 170.35 | 174.44 | |
| 0.4 | 176.61 | 0.994 | 1.57 × 1014 | 171.21 | 173.57 | 175.10 | 0.993 | 1.18 × 1014 | 169.70 | 175.12 | |
| 0.5 | 175.99 | 0.992 | 1.40 × 1014 | 170.59 | 174.20 | 174.31 | 0.991 | 1.01 × 1014 | 168.91 | 175.93 | |
| 0.6 | 184.22 | 0.977 | 6.71 × 1014 | 178.82 | 165.73 | 182.80 | 0.974 | 5.12 × 1014 | 177.40 | 167.20 | |
| 0.7 | 199.20 | 0.970 | 1.16 × 1016 | 193.80 | 150.34 | 198.26 | 0.967 | 9.71 × 1015 | 192.86 | 151.31 | |
| Average | 180.80 | 2.14 × 1015 | 175.40 | 169.25 | 179.44 | 1.77 × 1015 | 174.04 | 170.66 | |||
| HSD-CaO | 0.2 | 191.25 | 0.980 | 2.18 × 1015 | 185.82 | 160.09 | 190.89 | 0.977 | 2.04 × 1015 | 185.46 | 160.46 |
| 0.3 | 193.01 | 0.991 | 3.04 × 1015 | 187.58 | 158.28 | 192.48 | 0.990 | 2.75 × 1015 | 187.05 | 158.82 | |
| 0.4 | 192.95 | 0.998 | 3.00 × 1015 | 187.52 | 158.34 | 192.25 | 0.998 | 2.63 × 1015 | 186.82 | 159.06 | |
| 0.5 | 192.68 | 0.999 | 2.86 × 1015 | 187.25 | 158.62 | 191.81 | 0.999 | 2.42 × 1015 | 186.39 | 159.51 | |
| 0.6 | 200.26 | 0.996 | 1.20 × 1016 | 194.84 | 150.83 | 199.62 | 0.995 | 1.062 × 1016 | 194.19 | 151.49 | |
| 0.7 | 208.49 | 0.970 | 5.69 × 1016 | 203.06 | 142.39 | 207.98 | 0.966 | 5.16 × 1016 | 202.55 | 142.92 | |
| Average | 196.44 | 1.33 × 1016 | 191.01 | 154.76 | 195.84 | 1.20 × 1016 | 190.41 | 155.37 | |||
| Wave Number Range (cm−1) | Functional Group | Possible Species |
|---|---|---|
| 3750–3500 | O-H stretching | H2O |
| 3050–2700 | C-H stretching | CH4 |
| 2400–2250 | C-O stretching | CO2 |
| 2250–2050 | CO stretching | CO |
| 1800–1650 | C=O stretching | Carboxylic acids, Ketones, Aldehydes |
| 1600–1450 | C-C stretching, Benzene skeletal | Aromatics skeletal |
| 1425–1305 | C-H bending | Alkanes |
| 1300–1000 | C-O stretching, O-H bending | Phenols, Alcohols, Ethers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chen, Z.; Wang, J.; Li, D.; Luo, L.; Liu, Z. Catalytic Pyrolysis of Hydrochar by Calcined Eggshells for Bioenergy Production: Improved Thermo-Kinetic Studies and Reduced Pollutant Emissions. Catalysts 2023, 13, 1297. https://doi.org/10.3390/catal13091297
Yang S, Chen Z, Wang J, Li D, Luo L, Liu Z. Catalytic Pyrolysis of Hydrochar by Calcined Eggshells for Bioenergy Production: Improved Thermo-Kinetic Studies and Reduced Pollutant Emissions. Catalysts. 2023; 13(9):1297. https://doi.org/10.3390/catal13091297
Chicago/Turabian StyleYang, Shengshu, Zeliang Chen, Jiaxiao Wang, Dong Li, Lei Luo, and Zhengang Liu. 2023. "Catalytic Pyrolysis of Hydrochar by Calcined Eggshells for Bioenergy Production: Improved Thermo-Kinetic Studies and Reduced Pollutant Emissions" Catalysts 13, no. 9: 1297. https://doi.org/10.3390/catal13091297