Cu/CuO-Decorated Peanut-Shell-Derived Biochar for the Efficient Degradation of Tetracycline via Peroxymonosulfate Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared Cu/CuO-BC Catalysts
2.1.1. Micromorphology and Specific Surface Area
2.1.2. Phase Structure and Composition
2.1.3. Surface Functional Groups
2.2. Catalytic Performance of Cu/CuO-BC
2.2.1. The Effect of Pyrolysis Temperature
2.2.2. The Recyclability of Cu/CuO-BC
2.2.3. The Effect of Co-existing Anions and Humic Acid
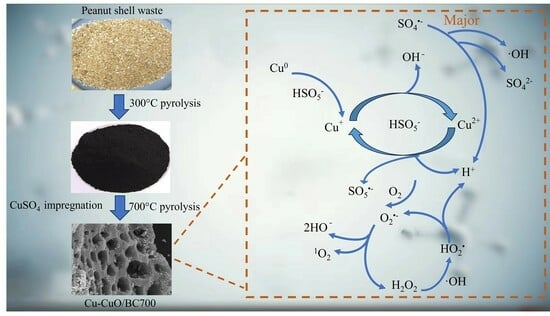
2.3. Mechanism of TC Degradation by PMS Activation with Cu/CuO-BC
2.3.1. Contribution of Active Species for TC Degradation
2.3.2. The Possible Generation Mechanism of Active Species
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Cu/CuO-BC Catalysts
3.3. Characterization
3.4. TC Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Serra, A.; Gómez, E.; Michler, J.; Philippe, L. Facile cost-effective fabrication of Cu@Cu2O@CuO-microalgae photocatalyst with enhanced visible light degradation of tetracycline. Chem. Eng. J. 2021, 413, 127477. [Google Scholar] [CrossRef]
- Huang, X.; Chen, C.; Zeng, Q.; Ding, D.; Gu, J.; Mo, J. Field study on loss of tetracycline antibiotics from manure-applied soil and their risk assessment in regional water environment of Guangzhou, China Sci. Total Environ. 2022, 827, 154273. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.A.; Polk, J.S.; Datta, T.; Parekh, R.R.; Agga, G.E. Occurrence of antibiotic resistant bacteria in urban karst groundwater systems. Water 2022, 14, 960. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Zang, J.; Li, Y.; Dong, X.; Jiang, F.; Wang, N.; Jiang, L.; Jiang, Q.; Fu, C. Estimates of dietary exposure to antibiotics among a community population in East China. Antibiotics 2022, 11, 407. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Żyłła, R.; Ledakowicz, S.; Boruta, T.; Olak-Kucharczyk, M.; Foszpańczyk, M.; Mrozińska, Z.; Balcerzak, J. Removal of tetracycline oxidation products in the nanofiltration process. Water 2021, 13, 555. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous advanced oxidation processes: Current approaches for wastewater treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The impact of tetracycline pollution on the aquatic environment and removal strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, K.; Xu, G.; Liang, B.; Yin, Q. Enhanced removal of tetracycline via advanced oxidation of sodium persulfate and biochar adsorption. Environ. Sci. Pollut. R. 2022, 29, 72556–72567. [Google Scholar] [CrossRef]
- Hao, D.; Chen, Y.; Zhang, Y.; You, N. Nanocomposites of zero-valent iron@ biochar derived from agricultural wastes for adsorptive removal of tetracyclines. Chemosphere 2021, 284, 131342. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Zielińska, I.; Szwast, M.; Kogut, I.; Małolepszy, A. Modification of ceramic membranes with carbon compounds for pharmaceutical substances removal from water in a filtration-Adsorption system. Membranes 2021, 11, 481. [Google Scholar] [CrossRef]
- Wang, X.; Jing, J.; Zhou, M.; Dewil, R. Recent advances in H2O2-based advanced oxidation processes for removal of antibiotics from wastewater. Chin. Chem. Lett. 2023, 34, 107621. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Li, D.; Sun, Y.; Ren, J.; Sun, J.; Yang, D. Theoretical study of local S coordination environment on Fe single atoms for peroxymonosulfate-based advanced oxidation processes. J. Hazard. Mater. 2023, 454, 131469. [Google Scholar] [CrossRef]
- Manos, D.; Miserli, K.; Konstantinou, I. Perovskite and spinel catalysts for sulfate radical-based advanced oxidation of organic pollutants in water and wastewater systems. Catalysts 2020, 10, 1299. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Liu, D.; Yi, M.; Chang, F.; Li, H.; Du, Y. A Review of Sulfate Radical-Based and Singlet Oxygen-Based Advanced Oxidation Technologies: Recent Advances and Prospects. Catalysts 2022, 12, 1092. [Google Scholar] [CrossRef]
- Li, Y.; Shang, H.; Cao, Y.; Yang, C.; Feng, Y.; Yu, Y. High performance removal of sulfamethoxazole using large specific area of biochar derived from corncob xylose residue. Biochar 2022, 4, 11. [Google Scholar] [CrossRef]
- Ghanbari, F.; Jaafarzadeh, N. Graphite-supported CuO catalyst for heterogeneous peroxymonosulfate activation to oxidize Direct Orange 26: The effect of influential parameters. Res. Chem. Intermed. 2017, 43, 4623–4637. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, D.; Liu, M.; Zhao, X. Insights into heterogeneous catalytic activation of peroxymonosulfate by Pd/g-C3N4: The role of superoxide radical and singlet oxygen. Catal. Commun. 2017, 102, 85–88. [Google Scholar] [CrossRef]
- Feng, M.; Xu, Z.; Bai, X.; Lin, K.; Zhang, M. Exploration the mechanisms underlying peroxymonosulfate activation by nano-cubic spinel M2MnO4 nanoparticles for degrading trichloroethylene. Chem. Eng. J. 2022, 446, 137394. [Google Scholar] [CrossRef]
- Manos, D.; Papadopoulou, F.; Margellou, A.; Petrakis, D.; Konstantinou, I. Heterogeneous activation of persulfate by LaMO3 (M= Co, Fe, Cu, Mn, Ni) perovskite catalysts for the degradation of organic compounds. Catalysts 2022, 12, 187. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Wu, J.; Miao, L. Mesoporous carbon framework supported Fe-Cu-Mn oxides as an efficient peroxymonosulfate catalyst for the control of harmful algal blooms: Synergism of Fe-Cu-Mn and role of mesoporous carbon. Chem. Eng. J. 2023, 461, 141877. [Google Scholar] [CrossRef]
- Ding, Y.; Li, D.; Zuo, S.; Guan, Z.; Ding, S. Boron-doping accelerated Cu(II)/Cu(I) cycle for enhancing peroxymonosulfate activation. Sep. Purif. Technol. 2022, 282, 120086. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Barsbay, M.; Zdorovets, M.V.; Zheltov, D.A.; Güven, O. Cu/CuO composite track-Etched membranes for catalytic decomposition of nitrophenols and removal of As (III). Nanomaterials 2020, 10, 1552. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Liu, L.; Duan, L.; Ren, Z.; Xu, S.; Chen, L.; Guo, H.; Huang, Y.; Shi, L.; et al. Cu/CuOx@C composite as a high-eficiency electrocatalyst for oxygen reduction reactions. Catalysts 2022, 12, 1515. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Qi, K.; Cui, X. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 2017, 7, 39695. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Zhou, Q.; Lee, S.L.J. A mini review on persulfate activation by sustainable biochar for the removal of antibiotics. Materials 2022, 15, 5832. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Zhu, C.; Aoki, Y.; Habazaki, H. Heteroatom-doped porous carbon with tunable pore structure and high specific surface area for high performance supercapacitors. Electrochim. Acta 2019, 314, 173–187. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Masood, I.; Zhou, B.; Wang, Y.; Lin, J.; Qiao, J.; Zhang, F. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction. Appl. Catal. B-Environ. 2020, 264, 118447. [Google Scholar] [CrossRef]
- Liang, Y.C.; Li, T.H. Sputtering-assisted synthesis of copper oxide–titanium oxide nanorods and their photoactive performances. Nanomaterials 2022, 12, 2634. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1, 4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass Bioenerg. 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Liang, G.; Wang, Z.; Yang, X.; Qin, T.; Xie, X.; Zhao, J.; Li, S. Efficient removal of oxytetracycline from aqueous solution using magnetic montmorillonite-biochar composite prepared by one step pyrolysis. Sci. Total Environ. 2019, 695, 133800. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Wang, G.; Shen, J.; Wei, S.; Cai, D.; Liu, J. Identification of Heavy Metals and Organic Micropollutants in Drinking Water Sources in Typical Villages and Towns in Northeast China. Molecules 2022, 27, 8033. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Fang, G.; Wang, Y.; Zhou, D. Mechanistic understanding of polychlorinated biphenyls degradation by peroxymonosulfate activated with CuFe2O4 nanoparticles: Key role of superoxide radicals. Chem. Eng. J. 2018, 348, 526–534. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Yang, C. Efficient removal of ciprofloxacin by peroxymonosulfate/Mn3O4-MnO2 catalytic oxidation system. Chem. Eng. J. 2017, 327, 481–489. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.H.; Pignatello, J.J. Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Das, B.; Devi, M.; Deb, S.; Dhar, S.S. Boosting photocatalytic property of graphitic carbon nitride with metal complex fabrication for efficient degradation of organic pollutants. Chemosphere 2023, 323, 138230. [Google Scholar] [CrossRef]
- Nie, W.; Mao, Q.; Ding, Y.; Hu, Y.; Tang, H. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Zhong, H.; Zhang, H.; Xu, S.; Li, Y.; Wang, H.; Crittenden, J.C. Facilitating redox cycles of copper species by pollutants in peroxymonosulfate activation. Environ. Sci. Technol. 2022, 56, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Li, N.; Wang, S.; Yu, J.; Li, X. Efficient degradation of ciprofloxacin by magnetic γ-Fe2O3-MnO2 with oxygen vacancy in visible-light/peroxymonosulfate system. Chemosphere 2021, 276, 130257. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Li, H.; Wang, Y.; Yu, J.; Li, N.; Wang, S. Cu/CuO-Decorated Peanut-Shell-Derived Biochar for the Efficient Degradation of Tetracycline via Peroxymonosulfate Activation. Catalysts 2023, 13, 1246. https://doi.org/10.3390/catal13091246
Zhao J, Li H, Wang Y, Yu J, Li N, Wang S. Cu/CuO-Decorated Peanut-Shell-Derived Biochar for the Efficient Degradation of Tetracycline via Peroxymonosulfate Activation. Catalysts. 2023; 13(9):1246. https://doi.org/10.3390/catal13091246
Chicago/Turabian StyleZhao, Jianhui, Huan Li, Yuanzhou Wang, Jingjie Yu, Ning Li, and Shaopo Wang. 2023. "Cu/CuO-Decorated Peanut-Shell-Derived Biochar for the Efficient Degradation of Tetracycline via Peroxymonosulfate Activation" Catalysts 13, no. 9: 1246. https://doi.org/10.3390/catal13091246