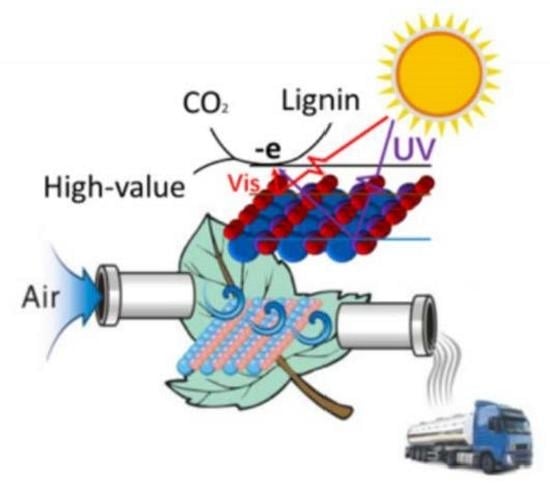
Facile Synthesis of Ni-Doped WO3-x Nanosheets with Enhanced Visible-Light-Responsive Photocatalytic Performance for Lignin Depolymerization into Value-Added Biochemicals
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Photocatalysts
3.3. Characterization
3.4. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 10. [Google Scholar]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.F.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Zhang, J.F.; Jiang, Y.; Easterling, L.F.; Anstner, A.; Li, W.R.; Alzarieni, K.Z.; Dong, X.M.; Bozell, J.; Kenttämaa, H.I. Compositional analysis of organosolv poplar lignin by using high-performance liquid chromatography/high-resolution multi-stage tandem mass spectrometry. Green Chem. 2021, 23, 983–1000. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, X.; Simmons, B.; Singh, S. Theory, practice and prospects of X-ray and neutron scattering for lignocellulosic biomass characterization: Towards understanding biomass pretreatment. Energy. Environ. Sci. 2015, 8, 436–455. [Google Scholar] [CrossRef]
- Hill, C.; Modification, W. Chemical, Thermal, and Other Processes; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Sun, Z.H.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Liu, M.; Lu, J.; Wang, F. Photo splitting of bio-polyols and sugars to methanol and syngas. Nat. Commun. 2020, 11, 1083. [Google Scholar] [CrossRef] [Green Version]
- Ugurlu, M.; Karaoglu, M.H. TiO2 supported on sepiolite: Preparation, structural and thermal characterization and catalytic behavior in photocatalytic treatment of phenol and lignin from olive mill wastewater. Chem. Eng. J. 2011, 166, 859–867. [Google Scholar] [CrossRef]
- Dai, J.; Patti, A.F.; Styles, G.N.; Nanayakkara, S.; Spiccia, L.; Arena, F.; Italiano, C.; Saito, K. Lignin oxidation by MnO2 under the irradiation of blue light. Green Chem. 2019, 21, 2005–2014. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021–17028. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Imbault, A.; Farnood, R. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination. Appl. Catal. B 2017, 204, 296–303. [Google Scholar] [CrossRef]
- Wu, X.J.; Li, J.Q.; Xie, S.J.; Duan, P.; Zhang, H.; Feng, J.; Zhang, Q.; Cheng, J.; Wang, Y. Selectivity control in photocatalytic valorization of biomass-derived platform compounds by surface engineering of titanium oxide. Chem 2020, 6, 3038–3053. [Google Scholar] [CrossRef]
- Wu, X.J.; Fan, X.T.; Xie, S.J.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Luo, N.C.; Wang, M.; Li, H.J.; Zhang, J.; Hou, T.; Chen, H.; Zhang, X.; Lu, J.; Wang, F. Visible-light-driven self-hydrogen transfer hydrogenolysis of lignin models and extracts into phenolic products. ACS Catal. 2017, 7, 4571–4580. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B.Z. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, K.; Li, P.; Jung, S.M.; Jeong, J.M.; Park, H.J. Dual oxygen and tungsten vacancies on a WO3 photoanode for enhanced water oxidation. Angew. Chem. Int. Edit. 2016, 55, 11819–11823. [Google Scholar] [CrossRef]
- Lu, Y.F.; Huang, Y.; Zhang, Y.F.; Huang, T.T.; Li, H.W.; Cao, J.J.; Ho, W.K. Effects of H2O2 generation over visible light-responsive Bi/Bi2O2−xCO3 nanosheets on their photocatalytic NOx removal performance. Chem. Eng. J. 2019, 363, 374–382. [Google Scholar] [CrossRef]
- Li, H.; Qin, F.; Yang, Z.P.; Cui, X.M.; Wang, J.F.; Zhang, L.Z. New reaction pathway induced by plasmon for selective benzyl alcohol oxidation on BiOCl possessing oxygen vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef]
- Peng, H.P.; Yang, T.; Lin, H.P.; Xu, Y.; Wang, Z.H.; Zhang, Q.H.; Liu, S.H.; Geng, H.B.; Gu, L.; Wang, C.; et al. Ru/In dual-single atoms modulated charge separation for significantly accelerated photocatalytic H2 evolution in pure water. Adv. Energy. Mater. 2022, 12, 2201688. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.J.; Li, K.; Yang, G.; Zhu, Y.L.; Liu, S.Q.; Lei, W. New reaction pathway induced by the synergistic effects of Bi plasmon and La3+ doping for efficient visible light photocatalytic reaction on BiOCl. Appl. Surf. Sci. 2018, 58, 769–780. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.Y.; Kim, K.J.; Kim, C.Y.; Jang, J.H.; Kim, Y.M.; Lee, H. Multiscale probing of the influence of the defect induced variation of oxygen vacancies on the photocatalytic activity of doped ZnO nanoparticle. J. Mater. Chem. A 2020, 8, 25345–25354. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.G.; Wang, J.; Hu, Z.W.; Shao, Q.; Xiao, X.H.; Huang, X.Q. Surface-regulated rhodium–antimony nanorods for nitrogen fixation. Angew. Chem. Int. Ed. 2020, 59, 8066–8071. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhao, Y.F.; Shi, R.; Wang, B.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Tuning oxygen vacancies in ultrathin TiO2 nanosheets to boost photocatalytic nitrogen fixation up to 700 nm. Adv. Mater. 2019, 31, 1806482. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, Z.X.; Li, Y.; Wang, J.T.; Wang, J.Q.; Zhang, G.K. Amorphous/crystalline Cu1.5Mn1.5O4 with rich oxygen vacancies for efficiently photothermocatalytic mineralization of toluene. Chem. Eng. J. 2023, 471, 144295. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Yang, L.Y.; Qiu, J.H.; Chen, Q.; Zhang, X.F.; Feng, Y.; Yao, J.F. Synergy of Ni dopant and oxygen vacancies in ZnO for efficient photocatalytic depolymerization of sodium lignosulfonate. Chem. Eng. J. 2020, 394, 125050. [Google Scholar] [CrossRef]
- Lu, C.H.; Li, X.R.; Wu, Q.; Li, J.; Wen, L.; Dai, Y.; Huang, B.B.; Li, B.J.; Lou, Z.Z. Constructing surface plasmon resonance on Bi2WO6 to boost high-selective CO2 reduction for methane. ACS Nano 2021, 15, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Wang, J.Q.; Wang, J.T.; Li, M.; Cheng, Q.; Wang, Z.Z.; Wang, X.T.; Li, J.M.; Li, Y.; Zhang, G.K. 2D WO3–x Nanosheet with rich oxygen vacancies for efficient visible-light-driven photocatalytic nitrogen fixation. Langmuir 2022, 3, 1178–1187. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhang, G.K.; Wang, K.; Wu, X.Y. Selective photocatalytic oxidation of low concentration methane over graphitic carbon nitride-decorated tungsten bronze cesium. ACS Sustain. Chem. Eng. 2019, 7, 4382–4389. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y.J. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893. [Google Scholar] [CrossRef]
- Shi, R.; Zhao, Y.X.; Waterhouse, G.I.N.; Zhang, S.; Zhang, T.R. Defect engineering in photocatalytic nitrogen fixation. ACS Catal. 2019, 9, 9739–9750. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wen, L.S.; Xi, J.B. Ni-Pd incorporated Fe3O4 yolk-shelled nanospheres as efficient magnetically recyclable catalysts for reduction of N-containing unsaturated compounds. Catalysts 2023, 13, 190. [Google Scholar] [CrossRef]
- Liang, C.; Niu, H.Y.; Guo, H.; Niu, C.G.; Huang, D.W.; Yan, Y.Y.; Liu, H.Y.; Shao, B.B.; Feng, H.P. Insight into photocatalytic nitrogen fixation on graphitic carbon nitride: Defect-dopant strategy of nitrogen defect and boron dopant. Chem. Eng. J. 2020, 396, 125395. [Google Scholar] [CrossRef]
- Huang, B.J.; Fu, X.X.; Wang, K.; Wang, L.; Zhang, H.L.; Liu, Z.Y.; Liu, B.; Li, J. Chemically bonded BiVO4/Bi19Cl3S27 heterojunction with fast hole extraction dynamics for continuous CO2 photoreduction. Adv. Powder Mater. 2023, 100140. [Google Scholar] [CrossRef]
- Wang, H.Y.; Niu, R.R.; Liu, J.H.; Guo, S.; Yang, Y.P.; Liu, Z.Y.; Li, J. Electrostatic self-assembly of 2D/2D CoWO4/g-C3N4 p-n heterojunction for improved photocatalytic hydrogen evolution: Built-in electric field modulated charge separation and mechanism unveiling. Nano Res. 2022, 15, 6987–6998. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Li, Y.H.; Qi, M.Y.; Li, J.Y.; Tang, Z.R.; Zhou, Z.; Xu, Y.J. Transition metal doping BiOBr nanosheets with oxygen vacancy and exposed {102} facets for visible light nitrogen fixation. Chem. Eng. J. 2021, 281, 119516. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hai, X.; Ding, X.; Chang, K.; Xiang, Y.G.; Meng, X.G.; Yang, Z.X.; Chen, H.; Ye, J.H. Light-switchable oxygen vacancies in ultrafine Bi5O7Br nanotubes for boosting solar-driven nitrogen fixation in pure water. Adv. Mater. 2017, 29, 1701774. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, T.; Zhao, Z.J.; Yang, W.M.; Li, J.F.; Li, A.; Yang, Z.L.; Ozin, G.A.; Gong, J.L. Promoted fixation of molecular nitrogen with surface oxygen vacancies on plasmon-enhanced TiO2 photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wang, H.D.; Tang, R.; Cheng, Q.; Yuan, Y.J. Rutile TiO2 nanoparticles with oxygen vacancy for photocatalytic nitrogen fixation. ACS Appl. Nano Mater. 2021, 4, 8674–8679. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.Y.; Pan, W.F.; Zhang, G.K.; Chen, H. Vacancy-rich monolayer BiO2-x as highly efficient UV, visible and near-infrared responsive photocatalyst. Angew. Chem. Int. Ed. 2018, 57, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Li, J.; Xiang, Q.J.; Liu, Z.Y.; Liu, B. Identification of the active sites on metallic MoO2−x nano-sea-urchin for atmospheric CO2 photoreduction under UV, visible, and near-infrared light illumination. Agnew. Chem. Int. Ed. 2023, 62, e202213124. [Google Scholar] [CrossRef]
- Niu, R.R.; Liu, Q.Y.; Huang, B.J.; Liu, Z.Y.; Zhang, W.F.; Peng, Z.K.; Wang, Z.Y.; Yang, Y.P.; Gu, Z.K.; Li, J. Black phosphorus/Bi19Br3S27 van der Waals heterojunctions ensure the supply of activated hydrogen for effective CO2 photoreduction. Appl. Catal. B 2022, 317, 121727. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Y.; Xiao, X. Facile Synthesis of Ni-Doped WO3-x Nanosheets with Enhanced Visible-Light-Responsive Photocatalytic Performance for Lignin Depolymerization into Value-Added Biochemicals. Catalysts 2023, 13, 1205. https://doi.org/10.3390/catal13081205
Wang H, Li Y, Xiao X. Facile Synthesis of Ni-Doped WO3-x Nanosheets with Enhanced Visible-Light-Responsive Photocatalytic Performance for Lignin Depolymerization into Value-Added Biochemicals. Catalysts. 2023; 13(8):1205. https://doi.org/10.3390/catal13081205
Chicago/Turabian StyleWang, Hao, Yuan Li, and Xintong Xiao. 2023. "Facile Synthesis of Ni-Doped WO3-x Nanosheets with Enhanced Visible-Light-Responsive Photocatalytic Performance for Lignin Depolymerization into Value-Added Biochemicals" Catalysts 13, no. 8: 1205. https://doi.org/10.3390/catal13081205