Synthesis of Ternary Cross-Linked MoS2/WS2/CdS Photocatalysts for Photocatalytic H2 Production
Abstract
:1. Introduction
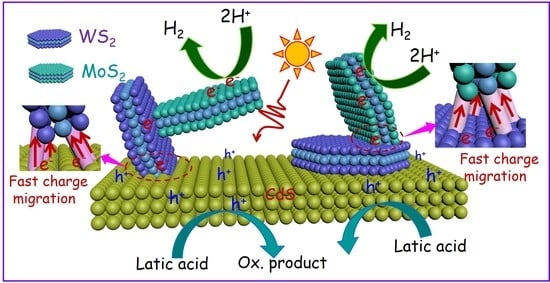
2. Results
3. Experimental
3.1. Preparation of Photocatalysts
3.1.1. Preparation of 1T-MoS2
3.1.2. Preparation of MoS2/WS2
3.1.3. Preparation of MoS2/WS2/CdS
3.2. Characterization
3.3. Photoelectrochemical Measurements
3.4. Photocatalytic Performance Evaluation System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chi, J.; Jiang, Z.; Yan, J.; Larimi, A.; Wang, Z.; Wang, L.; Shangguan, W. Recent advancements in bismuth vanadate photoanodes for photoelectrochemical water splitting. Mater. Today Chem. 2022, 26, 101060. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Z.; Cheng, X.; Shi, W. Research Progress of ZnIn2S4-Based Catalysts for Photocatalytic Overall Water Splitting. Catalysts 2023, 13, 967. [Google Scholar] [CrossRef]
- He, Y.; Dai, X.; Ma, S.; Chen, L.; Feng, Z.; Xing, P.; Yu, J.; Wu, Y. Hydrothermal preparation of carbon modified KNb3O8 nanosheets for efficient photocatalytic H2 evolution. Ceram. Int. 2020, 46, 11421–11426. [Google Scholar] [CrossRef]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.; Zhang, P.; Li, X. Nanostructured CdS for efficient photocatalytic H2 evolution: A review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Mirsalari, S.A.; Nezamzadeh-Ejhieh, A.; Massah, A.R. A designed experiment for CdS-AgBr photocatalyst toward methylene blue. Environ. Sci. Pollut. Res. 2022, 29, 33013–33032. [Google Scholar] [CrossRef]
- Bie, C.; Fu, J.; Cheng, B.; Zhang, L. Ultrathin CdS nanosheets with tunable thickness and efficient photocatalytic hydrogen generation. Appl. Surf. Sci. 2018, 462, 606–614. [Google Scholar] [CrossRef]
- Cai, M.; Cao, S.; Zhuo, Z.; Wang, X.; Shi, K.; Cheng, Q.; Xue, Z.; Du, X.; Shen, C.; Liu, X.; et al. Fabrication of Ni2P Cocatalyzed CdS Nanorods with a Well-Defined Heterointerface for Enhanced Photocatalytic H2 Evolution. Catalysts 2022, 12, 417. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Li, N.; Ge, L. In-situ constructing cobalt incorporated nitrogen-doped carbon/CdS heterojunction with efficient interfacial charge transfer for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 27961–27972. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, Y.; Zhang, L.; Wang, A.; Xiao, N.; Gao, Y.; Li, N.; Song, W.; Ge, L.; et al. Novel photocatalyst incorporating Ni-Co layered double hydroxides with P-doped CdS for enhancing photocatalytic activity towards hydrogen evolution. Appl. Catal. B 2019, 254, 145–155. [Google Scholar] [CrossRef]
- Coogan, Á.; Gun’ko, Y.K. Solution-based “bottom-up” synthesis of group VI transition metal dichalcogenides and their applications. Mater. Adv. 2021, 2, 146–164. [Google Scholar] [CrossRef]
- Teo, W.Z.; Chng, E.L.; Sofer, Z.; Pumera, M. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem. Eur. J. 2014, 20, 9627–9632. [Google Scholar] [CrossRef] [PubMed]
- Anto Jeffery, A.; Nethravathi, C.; Rajamathi, M. Two-dimensional nanosheets and layered hybrids of MoS2 and WS2 through exfoliation of ammoniated MS2 (M = Mo, W). J. Phys. Chem. C 2014, 118, 1386–1396. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, C.; Cao, X.; Zhang, L.; Chen, T.; Guo, C.; Wang, J. Synthesis of CdS/CoP hollow nanocages with improved photocatalytic water splitting performance for hydrogen evolution. J. Environ. Chem. Eng. 2021, 9, 106270. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical layered WS2/graphene-modified CdS nanorods for efficient photocatalytic hydrogen evolution. ChemSusChem 2016, 9, 996–1002. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Jiang, S. Horizontally growth of WS2/WO3 heterostructures on crystalline g-C3N4 nanosheets towards enhanced photo/electrochemical performance. J. Nanostruct. Chem. 2021, 11, 367–380. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Qiao, X.; Hou, D.; Li, D. One-pot hydrothermal synthesis of willow branch-shaped MoS2/CdS heterojunctions for photocatalytic H2 production under visible light irradiation. Chin. J. Catal. 2019, 40, 371–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xin, X.; Song, Y.; Yang, L.; Wang, B.; Tan, L.; Li, X. Plasmonic MoO2 as co-catalyst of MoS2 for enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 504, 144291. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, C.; Cheng, S.; Yang, X. Ultrahigh photocatalytic hydrogen evolution performance of coupled 1D CdS/1T-phase dominated 2D WS2 nanoheterojunctions. Chin. J. Catal. 2022, 43, 403–409. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Hong, S.; Kumar, D.P.; Kim, T. Hydrazine-assisted formation of ultrathin MoS2 nanosheets for enhancing their co-catalytic activity in photocatalytic hydrogen evolution. J. Mater. Chem. A 2017, 5, 6981–6991. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Jing, J.; Sun, M.; Tian, J.; Lu, G.; Ma, L.; Li, X.; Hou, J. Significantly enhanced photocatalytic hydrogen production performance of g-C3N4/CNTs/CdZnS with carbon nanotubes as the electron mediators. J. Mater. Sci. Technol. 2021, 80, 75–83. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, S.; Wang, X.; Cui, H.; Wang, X.; Tian, J. The metallic 1T-phase WS2 nanosheets as cocatalysts for enhancing the photocatalytic hydrogen evolution of g-C3N4 nanotubes. Appl. Catal. B 2020, 274, 119114. [Google Scholar] [CrossRef]
- Su, L.; Luo, L.; Song, H.; Wu, Z.; Tu, W.; Wang, Z.; Ye, J. Hemispherical shell-thin lamellar WS2 porous structures composited with CdS photocatalysts for enhanced H2 evolution. Chem. Eng. J. 2020, 388, 124346. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Wei, S.; Heng, Q.; Wang, B.; Liu, S.; Gao, L.; Mao, L. In situ growth of a-few-layered MoS2 on CdS nanorod for high efficient photocatalytic H2 production. Front. Energy 2021, 15, 752–759. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, M.; Seok, H.; Park, K.Y.; Moon, J.Y.; Park, J.; An, B.S.; Jung, H.; Dravid, V.; Whang, D.; et al. Realization of wafer-scale 1T-MoS2 film for efficient hydrogen evolution reaction. ChemSusChem 2021, 14, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Hu, Y.; Zhou, X.; Zhang, M.; Jia, X.; Lin, B.; Chen, G. One-step synthesis of 1T MoS2 hierarchical nanospheres for electrocatalytic hydrogen evolution. ACS Appl. Energy Mater. 2022, 5, 11705–11712. [Google Scholar] [CrossRef]
- Yi, J.; She, X.; Song, Y.; Mao, M.; Xia, K.; Xu, Y.; Mo, Z.; Wu, J.; Xu, H.; Li, H. Solvothermal synthesis of metallic 1T-WS2: A supporting co-catalyst on carbon nitride nanosheets toward photocatalytic hydrogen evolution. Chem. Eng. J. 2018, 335, 282–289. [Google Scholar] [CrossRef]
- Srinivaas, M.; Wu, C.Y.; Duh, J.G.; Wu, J. Highly rich 1T metallic phase of few-layered WS2 nanoflowers for enhanced storage of lithium-ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 10363–10370. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, S.J.; Kang, T.W.; Ju, J.M.; Yim, D.; Kim, H.I.; Park, J.; Kim, J.H. 2H-WS2 quantum dots produced by modulating the dimension and phase of 1T-nanosheets for antibody-free optical sensing of neurotransmitters. ACS Appl. Mater. Interfaces 2017, 9, 12316–12323. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the g-C3N4 interlayers for enhanced photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Heng, Q.; Wang, B.; Fan, X.; Chen, W.; Li, X.; Mao, L.; Shangguan, W. Enhanced photoreduction activity of CO2 to CO over Ag-loaded mesoporous g-C3N4 (MCN) by promoting charge separation and CO2 adsorption. J. Alloys Compd. 2022, 920, 165945. [Google Scholar] [CrossRef]
- Li, F.; Yang, J.; Gao, J.; Liu, Y.; Gong, Y. Enhanced photocatalytic hydrogen production of CdS embedded in cationic hydrogel. Int. J. Hydrogen Energy 2020, 45, 1969–1980. [Google Scholar] [CrossRef]
- Song, T.; Hou, L.; Long, B.; Ali, A.; Deng, G.J. Ultrathin MXene “bridge” to accelerate charge transfer in ultrathin metal-free 0D/2D black phosphorus/g-C3N4 heterojunction toward photocatalytic hydrogen production. J. Colloid Interface Sci. 2021, 584, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Shangguan, W. Metal (oxide) modified (M = Pd, Ag, Au and Cu) H2SrTa2O7 for photocatalytic CO2 reduction with H2O: The effect of cocatalysts on promoting activity toward CO and H2 evolution. Int. J. Hydrogen Energy 2019, 44, 4123–4132. [Google Scholar] [CrossRef]
- Heng, Q.; Ma, Y.; Wang, X.; Wu, Y.; Li, Y.; Chen, W. Role of Ag, Pd cocatalysts on layered SrBi2Ta2O9 in enhancing the activity and selectivity of photocatalytic CO2 reaction. Appl. Surf. Sci. 2023, 632, 157564. [Google Scholar] [CrossRef]
- Lu, X.; Chen, W.; Yao, Y.; Wen, X.; Hart, J.; Tsounis, C.; Toe, C.; Scott, J.; Ng, Y. Photogenerated charge dynamics of CdS nanorods with spatially distributed MoS2 for photocatalytic hydrogen generation. Chem. Eng. J. 2021, 420, 127709. [Google Scholar] [CrossRef]
- Chen, R.; Wang, P.; Chen, J.; Wang, C.; Ao, Y. Synergetic effect of MoS2 and MXene on the enhanced H2 evolution performance of CdS under visible light irradiation. Appl. Surf. Sci. 2019, 473, 11–19. [Google Scholar] [CrossRef]
- Li, L.; Gao, D.; Chen, F.; Wang, X.; Yu, H. Amorphization-crystallization synergism on MoSx homojunction for boosting photocatalytic H2 production of TiO2 in alkaline medium. Appl. Surf. Sci. 2023, 608, 155173. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Lin, Y.; Liu, Q.; Qi, Y.; Pan, W.; He, P.; Qi, X.; Wang, R.; Ji, Z. Solvent-exfoliation of transition-metal dichalcogenide MoS2 to provide more active sites for enhancing photocatalytic performance of BiOIO3/g-C3N4 photocatalyst. Appl. Surf. Sci. 2019, 481, 838–851. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Fan, X.; Chen, W.; Liu, X.; Gao, L.; Mao, L. In situ growth of 1T-WS2 on ultrathin Ti3C2Tx as a hybrid cocatalyst for enhancing the photocatalytic activity of CdS. Appl. Surf. Sci. 2023, 615, 156305. [Google Scholar] [CrossRef]
- Fan, X.; Wang, B.; Heng, Q.; Chen, W.; Mao, L. Facile in-situ synthesis of a-NiS/CdS p-n junction with enhanced photocatalytic H2 production activity. Int. J. Hydrogen Energy 2022, 47, 32531–32542. [Google Scholar] [CrossRef]








| Wavelength | I (W·cm−1) | nH2 (μmol·h−1) | Np a | NH b | AQY |
|---|---|---|---|---|---|
| 420 nm | 21.73 | 178.3 | 1.01 × 1017 | 5.96 × 1016 | 58.9% |
| 450 nm | 40.15 | 213.1 | 2.00 × 1017 | 7.13 × 1016 | 35.6% |
| 470 nm | 44.91 | 205.1 | 2.36 × 1017 | 6.86 × 1016 | 29.0% |
| 520 nm | 38.44 | 157.6 | 2.22 × 1017 | 5.27 × 1016 | 23.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, B.; Liu, X.; Gao, L.; Shangguan, W. Synthesis of Ternary Cross-Linked MoS2/WS2/CdS Photocatalysts for Photocatalytic H2 Production. Catalysts 2023, 13, 1149. https://doi.org/10.3390/catal13081149
Sun Y, Wang B, Liu X, Gao L, Shangguan W. Synthesis of Ternary Cross-Linked MoS2/WS2/CdS Photocatalysts for Photocatalytic H2 Production. Catalysts. 2023; 13(8):1149. https://doi.org/10.3390/catal13081149
Chicago/Turabian StyleSun, Yuping, Binfen Wang, Xiaoqiang Liu, Li Gao, and Wenfeng Shangguan. 2023. "Synthesis of Ternary Cross-Linked MoS2/WS2/CdS Photocatalysts for Photocatalytic H2 Production" Catalysts 13, no. 8: 1149. https://doi.org/10.3390/catal13081149