Hydrothermally Derived Mg-Doped TiO2 Nanostructures for Enhanced H2 Evolution Using Photo- and Electro-Catalytic Water Splitting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Powder X-ray Diffraction (PXRD) Studies
2.2. Transmission Electron Microscopy (TEM) Studies
2.3. Field Emission Scanning Electron Microscopy (FESEM) Studies
2.4. Raman Studies
2.5. BET Surface Area Studies
2.6. Photocatalytic Water Splitting for H2 Evolution Studies
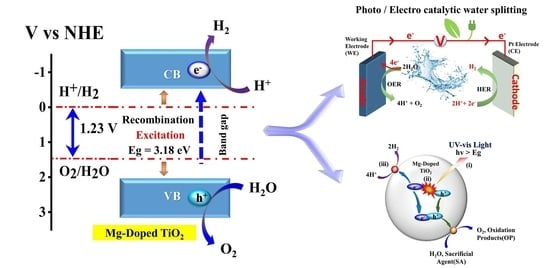
2.7. Possible Photocatalytic Reaction Mechanism of Mg-Doped TiO2 Nanoparticles
2.8. Electrocatalytic Water Splitting Studies
3. Experimental Section
3.1. Materials Required
3.2. Synthesis of Mg-Doped TiO2 Nanoparticles
3.3. Characterizations
3.4. Photocatalytic H2 Evolution Measurements
3.5. Electrode Preparation and Electrocatalytic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadiq, I.; Ali, S.A.; Ahmad, T. Graphene-based derivatives heterostructured catalytic systems for sustainable hydrogen energy via overall water splitting. Catalysts 2023, 13, 109. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Liu, J.; Guo, C. Engineering transition metal-based nanomaterials for high-performance electrocatalysis. Mater. Rep. Energy 2021, 1, 100006. [Google Scholar] [CrossRef]
- Pandit, M.A.; Billakanti, S.; Muralidharan, K. A simplistic approach for the synthesis of CuS-CdS heterostructure: A novel photocatalyst for oxidative dye degradation. J. Environ. Chem. Eng. 2020, 8, 103542. [Google Scholar] [CrossRef]
- Bergamini, L.; Sangiorgi, N.; Gondolini, A.; Sanson, A. CsPbBr3 for photoelectrochemical cells. Sol. Energy 2020, 212, 62–72. [Google Scholar] [CrossRef]
- Fazil, M.; Ahmad, T. Pristine TiO2 and Sr-doped TiO2 nanostructures for enhanced photocatalytic and electrocatalytic water splitting applications. Catalysts 2023, 13, 93. [Google Scholar] [CrossRef]
- Khan, H.; Lone, I.H.; Lofland, S.E.; Ramanujachary, K.V.; Ahmad, T. Exploiting multiferroicity of TbFeO3 nanoparticles for hydrogen generation through photo/electro/photoelectro-catalytic watersplitting. Int. J. Hydrogen Energy 2023, 48, 5493–5505. [Google Scholar] [CrossRef]
- Li, M.; Song, W.; Zeng, L.; Zeng, D.; Xie, C.; Yang, Q. Mechanistic study of N–H- and H–N-codoping of a TiO2 photocatalyst for efficient degradation of benzene under visible light. RSC Adv. 2020, 10, 2757–2766. [Google Scholar] [CrossRef]
- Pandit, N.A.; Ahmad, T. Tin oxide based hybrid nanostructures for efficient gas sensing. Molecules 2022, 27, 7038. [Google Scholar] [CrossRef]
- Hameed, R.A. Nanostructured phosphides as electrocatalysts for green energy generation in noble metal-free electrocatalysts: New trends in electrocatalysts for energy applications. J. Am. Chem. Soc. 2022, 2, 191–235. [Google Scholar]
- Srinivas, B.; Pandit, M.A.; Muralidharan, K. Importance of clean surfaces on the catalyst: SnS2 nanorings for environmental remediation. ACS Omega 2019, 4, 14970–14980. [Google Scholar] [CrossRef]
- Wealer, B.; Seidel, J.P.; Hirschhausen, C. Decommissioning of nuclear power plants and storage of nuclear waste: Experiences from Germany, France, and the UK. In Technological and Economic Future of Nuclear Power; Springer: Berlin/Heidelberg, Germany, 2019; pp. 261–286. [Google Scholar]
- Gao, L.; Li, Y.; Ren, J.; Wang, S.; Wang, R.; Fu, G.; Hu, Y. Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: Realizing efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Baum, Z.J.; Diaz, L.L.; Konovalova, T.; Zhou, Q.A. Materials research directions toward a green hydrogen economy: A review. ACS Omega 2022, 7, 32908–32935. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Sadiq, I.; Ali, S.A.; Ahmad, T. Bismuth-based multi-component heterostructured nanocatalysts for hydrogengeneration. Catalysts 2023, 13, 295. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Chemical strategies in molybdenum based chalcogenides nanostructures for photocatalysis. Int. J. Hydrogen Energy 2022, 29, 29255–29283. [Google Scholar] [CrossRef]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/graphitic carbon nitride heterojunctions for high-efficiency photocatalytic and electrocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2022, 14, 44317–44329. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Sadiq, I.; Ahmad, T. Oxide based heterostructured photocatalysts for CO2 reduction and hydrogen generation. ChemistrySelect 2023, 8, e202203176. [Google Scholar] [CrossRef]
- Khan, M.I.; Farooq, W.A.; Saleem, M.; Bhatti, K.A.; Atif, M.; Hanif, A. Phase change, bandgap energy and electrical resistivity of Mg doped TiO2 multilayer thin films for dye sensitized solar cells applications. Ceram. Int. 2019, 45, 21436–21439. [Google Scholar] [CrossRef]
- Jain, S.K.; Fazil, M.; Naaz, F.; Pandit, N.A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Silver-doped SnO2 nanostructures for photocatalytic watersplitting and catalytic nitrophenol reduction. New J. Chem. 2022, 46, 2846–2857. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Liang, Y.; Cui, W.; Wu, P. Tailoring band structure of TiO2 to enhance photoelectrochemical activity by codoping S and Mg. J. Phys. Chem. C 2015, 119, 11557–11562. [Google Scholar] [CrossRef]
- Nurlaela, E.; Ziani, A.; Takanabe, K. Tantalum nitride for photocatalytic watersplitting: Concept and applications. Mater. Renew. Sustain. Energy 2016, 5, 18. [Google Scholar] [CrossRef]
- Khan, H.; Mehtab, A.; Ahmed, J.; Lofland, S.E.; Ramanujachary, K.V.; Ahmad, T. Harvesting green hydrogen by self propelling built-in electric field photo/electro-catalytic performance of DyCrO3 nanoparticles developed by reverse microemulsion route. ChemNanoMat 2023, e202300091. [Google Scholar] [CrossRef]
- Li, P.; Kong, L.; Liu, J.; Yan, J.; Liu, S. Photoassisted hydrothermal synthesis of IrOx–TiO2 for enhanced water oxidation. ACS Sustain. Chem. Eng. 2019, 7, 17941–17949. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Herbaut, M.; Siaj, M.; Claverie, J.P. Nanomaterials-based water splitting: How far are we from a sustainable solution? ACS Appl. Nano Mater. 2021, 4, 907–910. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Hou, J.; Liu, S.; Qin, Y.; Liu, Q.; Cai, X.; Sun, Z.; Yang, M.; Luo, J.; et al. Cu2O-derived Pt Cu nanoalloy toward energy-efficient hydrogen production via hydrazine electrolysis under large current density. ACS Appl. Energy Mater. 2022, 5, 9487–9494. [Google Scholar] [CrossRef]
- Du, L.; Sun, Y.; You, B. Hybrid water electrolysis: Replacing oxygen evolution reaction for energy-efficient hydrogen production and beyond. Mater. Rep. Energy 2021, 1, 100004. [Google Scholar] [CrossRef]
- Mehtab, A.; Alshehri, S.M.; Ahmad, T. Photocatalytic and photoelectrocatalytic water splitting by porous g-C3N4 nanosheets for hydrogen generation. ACS Appl. Nano Mater. 2022, 5, 12656–12665. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous catalysts and electrochemical watersplitting: An untold story of harmony. Small 2020, 16, 1905779. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.C.; Venturini, S.I.; Zhang, S.; Löffler, M.; Scheu, C.; Mayrhofer, K.J.; Ticianelli, E.A.; Cherevko, S. Oxygen evolution reaction on tinoxides supported iridium catalysts: Do we need dopants? ChemElectroChem 2020, 7, 2330–2339. [Google Scholar]
- Vasu, D.; Keyan, A.K.; Sakthinathan, S.; Chiu, T. Investigation of electrocatalytic and photocatalytic ability of Cu/Ni/TiO2/MWCNTs nanocomposites for detection and degradation of antibiotic drug Furaltadone. Sci. Rep. 2022, 12, 886. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Enhanced hydrogen generation via overall water splitting using novel MoS2-BN nanoflowers assembled TiO2 ternary heterostructures. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Bergamini, L.; Sangiorgi, N.; Gondolini, A.; Rancan, M.; Bottaro, G.; Armelao, L.; Sanson, A. CsPbBr3/platinum and CsPbBr3/graphite hybrid photoelectrodes for carbondioxide conversion to oxalic acid. Sol. Energy 2023, 254, 213–222. [Google Scholar] [CrossRef]
- Naaz, F.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Unraveling the Chemoselective Catalytic, Photocatalytic and Electrocatalytic Applications of Copper supported WO3 Nanosheets. Catal. Commun. 2023, 178, 106678. [Google Scholar] [CrossRef]
- Vignesh, K.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar]
- Samuel, E.; Joshi, B.; Kim, M.; Swihart, M.T.; Yoon, S.S. Morphology engineering of photoelectrodes for efficient photoelectrochemical watersplitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmad, T. Investigating the Spatial Charge Density Flow and Molecular Structure of g-C3N4 Photocatalyst from a Computational Perspective. Appl. Catal. A Gen. 2023, 659, 119190. [Google Scholar] [CrossRef]
- Kim, H.J.; Jackson, D.H.; Lee, J.; Guan, Y.; Kuech, T.F.; Huber, G.W. Enhanced activity and stability of TiO2-coated cobalt/carbon catalysts for electrochemical wateroxidation. ACS Catal. 2015, 5, 3463–3469. [Google Scholar] [CrossRef]
- Lei, M.; Guo, S.; Wang, Z.; Zhu, L.; Tang, H. Ultra rapid and deep debromination of tetrabromodiphenylether over noble-metal-free Cu/TiO2 nanocomposites under mild conditions. Environ. Sci. Technol. 2018, 52, 11743–11751. [Google Scholar] [PubMed]
- Lee, C.G.; Javed, H.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P.J. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Elsenety, M.M.; Sadik, W.; El-Demerdash, A.; Nashed, A.; Mostafa, A.; Elyamny, S. The superior photocatalytic performance and DFT insights of S-scheme CuO@TiO2 heterojunction composites for simultaneous degradation of organics. Sci. Rep. 2022, 12, 2217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, P.; Meng, H.; Jiang, Z. Role of sulfites in the water splitting reaction. J. Solut. Chem. 2016, 45, 67–80. [Google Scholar] [CrossRef]
- Gautam, A.; Sk, S.; Pal, U. Recent advances in solution assisted synthesis of transition metal chalcogenides for photo-electrocatalytic hydrogen evolution. Phys. Chem. Chem. Phys. 2022, 24, 20638–20673. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Treasure trove for efficient hydrogen evolution through water splitting using diverse perovskite photocatalysts. Mater. Today Chem. 2023, 29, 101387. [Google Scholar] [CrossRef]
- Kavaliunas, V.; Krugly, E.; Sriubas, M.; Mimura, H.; Laukaitis, G.; Hatanaka, Y. Influence of Mg, Cu, and Ni dopants on amorphous TiO2 thin films photocatalytic activity. Materials 2020, 13, 886. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Hu, H.; Zeng, G.; Li, C. Recovering hydrogen energy from photocatalytic treatment of pharmaceutical-contaminated water using Co3O4 modified {001}/{101}-TiO2 nanosheets. ACS ES&T Eng. 2021, 1, 603–611. [Google Scholar]
- Athira, K.; Merin, K.T.; Raguram, T.; Rajni, K.S. Synthesis and characterization of Mg doped TiO2 nanoparticles for photocatalytic applications. Mater. Today Proc. 2020, 33, 2321–2327. [Google Scholar]
- Singh, S.V.; Kumar, M.P.; Anantharaj, S.; Mukherjee, B.; Kundu, S.; Pal, B.N. Direct evidence of an efficient plasmon-induced hot-electron transfer at an in situ grown Ag/TiO2 interface for highly enhanced solar H2 generation. ACS Appl. Energy Mater. 2020, 3, 1821–1830. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.; Moon, G.; Choi, W. Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Alizade, B.; Modirshahla, N. Synthesis of Mg-doped TiO2 nanoparticles under different conditions and its photocatalytic activity. Photochem. Photobiol. 2011, 87, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Malligavathy, M.; Iyyapushpam, S.; Nishanthi, S.T.; Padiyan, D.P. Photoreduction synthesis of silver on Bi2O3/TiO2 nanocomposites and their catalytic activity for the degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 18307–18321. [Google Scholar] [CrossRef]
- Giahi, M.; Pathania, D.; Agarwal, S.; Ali, G.A.; Chong, K.F.; Gupta, V.K. Preparation of Mg-doped TiO2 nanoparticles for photocatalytic degradation of some organic pollutants. Stud. UBB Chem. 2019, 64, 7–18. [Google Scholar] [CrossRef]
- Arshad, Z.; Khoja, A.H.; Shakir, S.; Afzal, A.; Mujtaba, M.A.; Soudagar, M.E.M.; Fayaz, H.; Farukh, S.; Saeed, M. Magnesium doped TiO2 as an efficient electron transport layer in perovskite solar cells. Case Stud. Therm. Eng. 2021, 26, 101101. [Google Scholar] [CrossRef]
- Le, T.S.; Hoa, T.H.; Truong, D.Q. Shape-controlled f-doped TiO2 nanocrystals for Mg-ion batteries. J. Electroanal. Chem. 2019, 848, 113293. [Google Scholar] [CrossRef]
- Tristantini, D.; Ibadurrohman, M. Photocatalytic hydrogen production from glycerol–water mixture over Pt-N-TiO2 nanotube photocatalyst. Int. J. Energy Res. 2013, 11, 1372–1381. [Google Scholar]
- Zhang, X.; Song, P.; Cui, X. Nitrogen-doped TiO2 photocatalysts synthesized from titanium nitride: Characterizations and photocatalytic hydrogen evolution performance. J. Adv. Oxid. Technol. 2013, 16, 131–136. [Google Scholar] [CrossRef]
- Kumar, M.K.; Bhavani, K.; Srinivas, B.; Kumar, S.N.; Sudhakar, M.; Naresh, G.; Venugopal, A. Nanostructured bismuth and nitrogen co-doped TiO2 as an efficient light harvesting photocatalyst under natural sunlight for the production of H2 by H2O splitting. Appl. Catal. A 2016, 515, 91–100. [Google Scholar] [CrossRef]
- Chen, D.; Gao, H.; Yao, Y.; Zhu, L.; Zhou, X.; Peng, X.; Zhang, X. Pd loading, Mn+ (n = 1, 2, 3) metal ions doped TiO2 nanosheets for enhanced photocatalytic H2 production and reaction mechanism. Int. J. Hydrogen Energy 2022, 47, 10250–10260. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Liu, Z.; Yang, X.; Naraginti, S.; Xu, X.; Wang, X. Visible light photocatalytic mineralization of 17α-ethinylestradiol (EE2) and hydrogen evolution over silver and strontium modified TiO2 nanoparticles: Mechanisms and phytotoxicity assessment. RSC Adv. 2018, 8, 4329–4339. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Self-assembled interwoven nanohierarchitectures of NaNbO3 and NaNb1−xTaxO3 (0.05 ≤ x ≤ 0.20): Synthesis, structural characterization, photocatalytic applications, and dielectric properties. ACS Omega 2022, 7, 16952–16967. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.K.; Vaidya, S.; Ahmad, T. Synthesis of nanocrystalline materials through reverse micelles: A versatile methodology for synthesis of complex metal oxides. Bull. Mater. Sci. 2008, 31, 415–419. [Google Scholar] [CrossRef]












| Sample | SBET/(m2 g−1) | Average Pore Size/Å | VTotal/(cm3 g−1) |
|---|---|---|---|
| 1% Mg-doped TiO2 | 152.9 | 16.95 | 0.317 |
| 2.5% Mg-doped TiO2 | 164.2 | 16.93 | 0.391 |
| 5% Mg-doped TiO2 | 198.1 | 17.08 | 0.449 |
| S. No. | Dopant | Synthesis Method | Parameters | Hydrogen Production | Ref. |
|---|---|---|---|---|---|
| 1. | Platinum, Nitrogen | Photodeposition method | 1150 mL Pyrex vessel, UV light | 3200 μmol | [59] |
| 2. | Nitrogen | Solid state/calcination method | UV-Vis light irradiation, Na2S/Na2SO3 | 18 μmol | [60] |
| 3. | Bismuth, Nitrogen | Sol-gel method | Solar light, methanol | 1800 μmol g−1 | [61] |
| 4. | Pd/0.2% K+ | Hydrothermal method | _ | 76.6 μmol h−1 | [62] |
| 5. | Strontium, silver | sol-gel method | 500 W Xe arc lamp | 49.4 μmol h−1 | [63] |
| 6. | Strontium | Hydrothermal method | 200 W, Hg-Xe arc lamp | 3.3 mmol h−1 | [5] |
| 7. | Magnesium | Hydrothermal method | 170 W, Hg-Xe arc lamp | 4.87 mmol h−1 | In this work |
| S. No. | Materials | HER | Ref. | |
|---|---|---|---|---|
| Overpotential (V) to Attain 10 mA/cm2 | Tafel Slope (mV/dec) | |||
| 1. | 1% Mg-doped TiO2 | 0.99 | 139.57 | This work |
| 2. | 2.5% Mg-doped TiO2 | 0.98 | 123.5 | This work |
| 3. | 5% Mg-doped TiO2 | 0.95 | 130.26 | This work |
| 4. | Pristine TiO2 | 1.00 | 133.33 | [5] |
| S. No. | Materials | HER | |
|---|---|---|---|
| Cathodic Current Density (mA/cm2) at −1.38 V | Tafel Slope (mV/dec) | ||
| 1. | 1% Mg-doped TiO2 | 0.436 | 208.73 |
| 2. | 2.5% Mg-doped TiO2 | 1.00 | 126.30 |
| 3. | 5% Mg-doped TiO2 | 0.786 | 187.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazil, M.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Hydrothermally Derived Mg-Doped TiO2 Nanostructures for Enhanced H2 Evolution Using Photo- and Electro-Catalytic Water Splitting. Catalysts 2023, 13, 893. https://doi.org/10.3390/catal13050893
Fazil M, Alshehri SM, Mao Y, Ahmad T. Hydrothermally Derived Mg-Doped TiO2 Nanostructures for Enhanced H2 Evolution Using Photo- and Electro-Catalytic Water Splitting. Catalysts. 2023; 13(5):893. https://doi.org/10.3390/catal13050893
Chicago/Turabian StyleFazil, Mohd, Saad M. Alshehri, Yuanbing Mao, and Tokeer Ahmad. 2023. "Hydrothermally Derived Mg-Doped TiO2 Nanostructures for Enhanced H2 Evolution Using Photo- and Electro-Catalytic Water Splitting" Catalysts 13, no. 5: 893. https://doi.org/10.3390/catal13050893