Visible-Light-Driven GO/Rh-SrTiO3 Photocatalyst for Efficient Overall Water Splitting
Abstract
:1. Introduction
2. Results and Discussions
2.1. Characterization of GO/Rh-STO Photocatalysts
2.2. The Crystal Structure and Chemical States Analysis
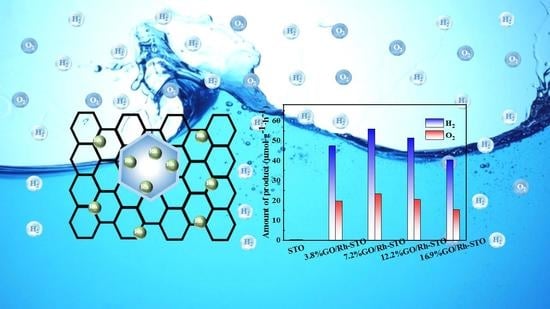
2.3. Photocatalytic Performance Evaluation
2.4. The Influence Factors of Enhancing Photocatalytic Performance
2.5. Photocatalytic Mechanism Research
3. Experiment
3.1. Materials
3.2. Synthesis and Preparation
3.2.1. Synthesis of Graphene Oxide (GO)
3.2.2. Synthesis of Strontium Titanate (STO)
3.2.3. Synthesis of GO/Rh-STO Composite Catalysts
3.3. Evaluation on Efficiency of Photocatalysts
3.4. Characterization
3.5. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Xu, M.; Xu, X.; Zhou, W.; Shao, Z. Perovskite oxide based electrodes for high-performance photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2020, 59, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Yang, Y. Graphitic carbon nitride for electrochemical energy conversion and storage. ACS Energy Lett. 2018, 3, 2796–2815. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, Z.; Wei, L.; Zhao, S.; Jian, X.; Chen, Y. Electrocatalytic hydrogen evolution under neutral pH conditions: Current understandings, recent advances, and future prospects. Energy Environ. Sci. 2020, 13, 3185–3206. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lu, G.; Yan, Z.; Wu, D.; Zhou, R.; Bao, X. Insights into a CQD-SnNb2O6/BiOCl Z-scheme system for the degradation of benzocaine: Influence factors, intermediate toxicity and photocatalytic mechanism. Chem. Eng. J. 2019, 374, 79–90. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]
- Tang, M.; Ao, Y.; Wang, C.; Wang, P. Rationally constructing of a novel dual Z-scheme composite photocatalyst with significantly enhanced performance for neonicotinoid degradation under visible light irradiation. Appl. Catal. B Environ. 2020, 270, 118918. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Zhang, X.; Wang, Y.; Liu, C.; Chen, G.; Dong, H. Insight into photocatalytic activity, universality and mechanism of copper/chlorine surface dual-doped graphitic carbon nitride for degrading various organic pollutants in water. J. Colloid Interface Sci. 2019, 538, 462–473. [Google Scholar] [CrossRef]
- Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Singh, A.A.P.K.; Asiri, A.M. Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and future scenario. J. Environ. Chem. Eng. 2020, 8, 103791. [Google Scholar] [CrossRef]
- Szafraniak, B.; Fusnik, Ł.; Xu, J.; Gao, F.; Brudnik, A.; Ry-dosz, A. Semiconducting metal oxides: SrTiO3, BaTiO3 and BaSrTiO3 in gas-sensing applications: A review. Coatings 2021, 11, 185. [Google Scholar] [CrossRef]
- Jayabal, P.; Sasirekha, V.; Mayandi, J.; Jeganathan, K.; Ramakrishnan, V. A facile hydrothermal synthesis of SrTiO3 for dye sensitized solar cell application. J. Alloys Compd. 2014, 586, 456–461. [Google Scholar] [CrossRef]
- Ghosh, D.; Giri, S.; Sahoo, S.; Das, C.K. In situ synthesis of graphene/amine-modified graphene, polypyrrole composites in presence of SrTiO3 for supercapacitor applications. Polym. Plast. Technol. Eng. 2013, 52, 213–220. [Google Scholar] [CrossRef]
- Muhamad, N.F.; Osman, R.A.M.; Idris, M.S.; Yasin, M.N.M. Physical and electrical properties of SrTiO3 and SrZrO3//EPJ Web of Conferences. EDP Sci. 2017, 162, 01052. [Google Scholar]
- Hu, X.J.; Yang, Y.; Hou, C.; Liang, T.X. Thermodynamic and Electronic Properties of Two-Dimensional SrTiO3. J. Phys. Chem. C 2021, 126, 517–524. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Sun, C.; Li, F.; Lu, G.Q.; Cheng, H.M. Synergistic effects of B/N doping on the visible-light photocatalytic activity of mesoporous TiO2. Angew. Chem. Int. Ed. 2008, 47, 4516–4520. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B Environ. 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, L.; Zhang, J.; Wang, F.; Xie, Y.; Shang, X.; Wang, X. In situ constructing interfacial contact MoS2/ZnIn2S4 heterostructure for enhancing solar photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 233, 112–119. [Google Scholar] [CrossRef]
- Maarisetty, D.; Baral, S.S. Defect engineering in photocatalysis: Formation, chemistry, optoelectronics, and interface studies. J. Mater. Chem. A 2020, 8, 18560–18604. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Putri, L.K.; Ong, W.J.; Chang, W.S.; Chai, S.P. Heteroatom doped graphene in photocatalysis: A review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Cui, D.; Hao, W.; Chen, J. The synergistic effect of heteroatom doping and vacancy on the reduction of CO2 by photocatalysts. ChemNanoMat 2021, 7, 894–901. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shi, Y.; Wang, Y.; Shiju, N.R. Nanocarbon catalysts: Recent understanding regarding the active sites. Adv. Sci. 2020, 7, 1902126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Zhang, H.; Fan, J.; Xiang, Q. Design and application of active sites in g-C3N4-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 69–88. [Google Scholar] [CrossRef]
- Ha, M.N.; Zhu, F.; Liu, Z.; Wang, L.; Liu, L.; Lu, G.; Zhao, Z. Morphology-controlled synthesis of SrTiO3/TiO2 heterostructures and their photocatalytic performance for water splitting. RSC Adv. 2016, 6, 21111–21118. [Google Scholar] [CrossRef]
- Deng, Y.; Shu, S.; Fang, N.; Wang, R.; Chu, Y.; Liu, Z.; Cen, W. One-pot synthesis of SrTiO3-SrCO3 heterojunction with strong interfacial electronic interaction as a novel photocatalyst for water splitting to generate H2. Chin. Chem. Lett. 2023, 34, 107323. [Google Scholar] [CrossRef]
- Pan, J.H.; Shen, C.; Ivanova, I.; Zhou, N.; Wang, X.; Tan, W.C.; Wang, Q. Self-template synthesis of porous perovskite titanate solid and hollow submicrospheres for photocatalytic oxygen evolution and mesoscopic solar cells. ACS Appl. Mater. Interfaces 2015, 7, 14859–14869. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Xu, Y.J. Recent progress on graphene-based photocatalysts: Current status and future perspectives. Nanoscale 2012, 4, 5792–5813. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.G. RGO–TiO2–ZnO composites: Synthesis, characterization, and application to photocatalysis. Appl. Catal. A Gen. 2015, 491, 52–57. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Sun, D.D.; Ng, W. Graphene oxide–CdS composite with high photocatalytic degradation and disinfection activities under visible light irradiation. J. Hazard. Mater. 2013, 250, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Hunge, Y.M.; Yadav, A.A.; Dhodamani, A.G.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V.L. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochem. 2020, 61, 104849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, Y.; Fu, Z.; Ren, J.; Fu, Y.; Wang, L. Construction of Ru/FeCoP heterointerface to drive dual active site mechanism for efficient overall water splitting. J. Mater. Chem. A 2022, 10, 16071–16079. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Muhamad Sarih, N.; Mohamad, S.; Joon Ching, J. SrTiO3 nanocube-doped polyaniline nanocomposites with enhanced photocatalytic degradation of methylene blue under visible light. Polymers 2016, 8, 27. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Kumar, N.A.; Choi, H.J.; Shin, Y.R.; Chang, D.W.; Dai, L.; Baek, J.B. Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano 2012, 6, 1715–1723. [Google Scholar] [CrossRef]
- Kiss, B.; Manning, T.D.; Hesp, D.; Didier, C.; Taylor, A.; Pickup, D.M.; Rosseinsky, M.J. Nano-structured rhodium doped SrTiO3–Visible light activated photocatalyst for water decontamination. Appl. Catal. B Environ. 2017, 206, 547–555. [Google Scholar] [CrossRef]
- Kawasaki, S.; Nakatsuji, K.; Yoshinobu, J.; Komori, F.; Takahashi, R.; Lippmaa, M.; Kudo, A. Epitaxial Rh-doped SrTiO3 thin film photocathode for water splitting under visible light irradiation. Appl. Phys. Lett. 2012, 101, 033910. [Google Scholar] [CrossRef]
- Kiran, K.S.; Ashwath Narayana, B.S.; Lokesh, S.V. Enhanced photocatalytic activity of perovskite SrTiO3 nanorods. Solid State Technol. 2020, 63, 1913–1920. [Google Scholar]
- Zhao, W.; Wang, H.; Liu, N.; Rong, J.; Zhang, Q.; Li, M.; Yang, X. Hydrothermal synthesis of Litchi-like SrTiO3 with the help of ethylene glycol. J. Am. Ceram. Soc. 2019, 102, 981–987. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; Tong, Z.; Nan, Y.; Jiang, Z. Fabrication of bimodal-pore SrTiO3 microspheres with excellent photocatalytic performance for Cr (VI) reduction under simulated sunlight. J. Hazard. Mater. 2016, 312, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Su, X.; Qing, D.; Zhao, Y.; Wang, J.; Zeng, X. Controlled synthesis of various SrTiO3 morphologies and their effects on photoelectrochemical cathodic protection performance. Ceram. Int. 2022, 48, 20228–20236. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Cui, H.; Wang, W.; Shang, Q.; Shi, X.; Tang, B. Photocatalytic overall water splitting by SrTiO3 with surface oxygen vacancies. Nanomaterials 2020, 10, 2572. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, J.; Yu, R.; Wan, J.; Wang, D. Constructing SrTiO3–TiO2 heterogeneous hollow multi-shelled structures for enhanced solar water splitting. Angew. Chem. Int. Ed. 2019, 58, 1422–1426. [Google Scholar] [CrossRef]
- Iwase, A.; Udagawa, Y.; Yoshino, S.; Ng, Y.H.; Amal, R.; Kudo, A. Solar Water Splitting under Neutral Conditions Using Z-Scheme Systems with Mo-Doped BiVO4 as an O2-Evolving Photocatalyst. Energy Technol. 2019, 7, 1900358. [Google Scholar] [CrossRef]
- Han, K.; Lin, Y.C.; Yang, C.M.; Jong, R.; Mul, G.; Mei, B. Promoting photocatalytic overall water splitting by controlled magnesium incorporation in SrTiO3 photocatalysts. ChemSusChem 2017, 10, 4510–4516. [Google Scholar] [CrossRef]
- Kanazawa, T.; Nozawa, S.; Lu, D.; Maeda, K. Structure and photocatalytic activity of PdCrOx cocatalyst on SrTiO3 for overall water splitting. Catalysts 2019, 9, 59. [Google Scholar] [CrossRef]
- Qureshi, M.; Garcia-Esparza, A.T.; Jeantelot, G.; Ould-Chikh, S.; Aguilar-Tapia, A.; Hazemann, J.L.; Takanabe, K. Catalytic consequences of ultrafine Pt clusters supported on SrTiO3 for photocatalytic overall water splitting. J. Catal. 2019, 376, 180–190. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, T.; Li, M.; Zeng, C.; Matsumoto, H.; Han, H. Water oxidation sites located at the interface of Pt/SrTiO3 for photocatalytic overall water splitting. Chin. J. Catal. 2022, 43, 2223–2230. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, X.; Shen, C.; Cai, M.; Jiang, Y.; Xue, Z.; Sun, S. Construction of TiO2/SrTiO3 Heterojunction Derived from Monolayer Ti3C2 MXene for Efficient Photocatalytic Overall Water Splitting. Chem. A Eur. J. 2023, 29, e202203450. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Chen, G.; Li, Z.H.; Zhang, Z.G. Electronic structure and visible light photocatalysis water splitting property of chromium-doped SrTiO3. J. Solid State Chem. 2006, 179, 3704–3708. [Google Scholar] [CrossRef]
- Ouyang, S.; Tong, H.; Umezawa, N.; Cao, J.; Li, P.; Bi, Y.; Ye, J. Surface-alkalinization-induced enhancement of photocatalytic H2 evolution over SrTiO3-based photocatalysts. J. Am. Chem. Soc. 2012, 134, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Liang, X.; Shi, H.; Wang, C.; Fan, J.; Liu, E. Enhanced photocatalytic activity of Ag-CsPbBr3/CN composite for broad spectrum photocatalytic degradation of cephalosporin antibiotics 7-ACA. Appl. Catal. B Environ. 2019, 247, 57–69. [Google Scholar] [CrossRef]
- Xu, B.; Li, Y.; Gao, Y.; Liu, S.; Lv, D.; Zhao, S.; Ge, L. Ag-AgI/Bi3O4Cl for efficient visible light photocatalytic degradation of methyl orange: The surface plasmon resonance effect of Ag and mechanism insight. Appl. Catal. B Environ. 2019, 246, 140–148. [Google Scholar] [CrossRef]
- Yu-Lei, D.; Guang, C.; Ming-Sheng, Z.; Sen-Zu, Y. Phonon characteristics of polycrystalline cubic SrTiO3 thin films. Chin. Phys. Lett. 2003, 20, 1561. [Google Scholar] [CrossRef]
- Rahman, J.U.; Du, N.V.; Nam, W.H.; Shin, W.H.; Lee, K.H.; Seo, W.S.; Lee, S. Grain boundary interfaces controlled by reduced graphene oxide in nonstoichiometric SrTiO3-δ thermoelectrics. Sci. Rep. 2019, 9, 8624. [Google Scholar] [CrossRef]
- Kogularasu, S.; Govindasamy, M.; Chen, S.M.; Akilarasan, M.; Mani, V. 3D graphene oxide-cobalt oxide polyhedrons for highly sensitive non-enzymatic electrochemical determination of hydrogen peroxide. Sens. Actuators B Chem. 2017, 253, 773–783. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Shi, G. Graphene based catalysts. Energy Environ. Sci. 2012, 5, 8848–8868. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Wang, X.; Dou, W. Preparation of graphite oxide (GO) and the thermal stability of silicone rubber/GO nanocomposites. Thermochim. Acta 2012, 529, 25–28. [Google Scholar] [CrossRef]
- Wei, X.; Xu, G.; Ren, Z.; Xu, C.; Weng, W.; Shen, G.; Han, G. Single-Crystal-like Mesoporous SrTiO3 Spheres with Enhanced Photocatalytic Performance. J. Am. Ceram. Soc. 2010, 93, 1297–1305. [Google Scholar]
- Wei, X.; Xu, G.; Ren, Z.; Xu, C.; Shen, G.; Han, G. PVA-Assisted Hydrothermal Synthesis of SrTiO3 Nanoparticles with Enhanced Photocatalytic Activity for Degradation of RhB. J. Am. Ceram. Soc. 2008, 91, 3795–3799. [Google Scholar] [CrossRef]









| GO Addition Amount (mg) | Atomic %C | Atomic %O | Atomic %Rh | Atomic %Sr | Atomic %Ti |
|---|---|---|---|---|---|
| 10 | 3.8 | 53.9 | 0.7 | 25.1 | 16.5 |
| 20 | 7.2 | 53.7 | 0.6 | 23.1 | 15.4 |
| 40 | 12.2 | 51.4 | 0.5 | 21.7 | 14.2 |
| 60 | 16.9 | 48.1 | 0.4 | 18.4 | 12.5 |
| Materials | Catalyst Dosage (mg) | Cocatalysts | Reactant Solution Water (mL) | Light Source | H2 Rate (μmol h−1·g−1) | O2 Rate (μmol h−1·g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| GO/Rh-STO | 100 | / | 100 | 300W Xe Lamp (λ > 420 nm) | 55.83 | 23.26 | This work |
| SrTiO3-C950 | 400 | / | 10 | 300W Xe Lamp (λ > 420 nm) | 2.3 | 1.0 | [44] |
| SrTiO3/TiO2 | 50 | Pt (0.3 wt%) | 150 | 300W Xe Lamp (λ > 420 nm) | 10.6 | 5.1 | [45] |
| SrTiO3:Rh-RGO-BiVO4:Mo | 200 | Ru (0.7 wt%) Co (0.1 wt%) | 120 | 300W Xe Lamp (λ > 420 nm) | 14 | 6.1 | [46] |
| Mg-doped SrTiO3 | 25 | Ni (1 wt%) | 25 | 300W Xe Lamp (λ > 300 nm) | 8.8 | 4.2 | [47] |
| PdCrOx/SrTiO3 | 100 | / | 140 | 300W Xe Lamp (λ > 300 nm) | 15 | 5 | [48] |
| Ultrafine Pt clusters on SrTiO3 | 50 | / | 100 | 300W Xe Lamp (λ > 300 nm) | 23 | 12 | [49] |
| SrTiO3 (impregnation methods) | 300 | Pt (0.3 wt%) | 150 | 300W Xe Lamp (λ > 300 nm) | 38 | 20 | [50] |
| TiO2/SrTiO3 | 100 | Rh (0.1 wt%) Cr (0.05 wt%) Co (0.05 wt%) | 50 | 300W Xe Lamp (λ < 380 nm) | 38.6 | 19.2 | [51] |
| oxygen vacancies SrTiO3 | 100 | Pt (0.3 wt%) | 60 | 300W Xe Lamp (λ < 380 nm) | 81 | 40 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Jiang, E.; Wu, J.; Liu, Z.; Yan, Y.; Huo, P.; Yan, Y. Visible-Light-Driven GO/Rh-SrTiO3 Photocatalyst for Efficient Overall Water Splitting. Catalysts 2023, 13, 851. https://doi.org/10.3390/catal13050851
Zhang S, Jiang E, Wu J, Liu Z, Yan Y, Huo P, Yan Y. Visible-Light-Driven GO/Rh-SrTiO3 Photocatalyst for Efficient Overall Water Splitting. Catalysts. 2023; 13(5):851. https://doi.org/10.3390/catal13050851
Chicago/Turabian StyleZhang, Shuai, Enhui Jiang, Ji Wu, Zhonghuan Liu, Yan Yan, Pengwei Huo, and Yongsheng Yan. 2023. "Visible-Light-Driven GO/Rh-SrTiO3 Photocatalyst for Efficient Overall Water Splitting" Catalysts 13, no. 5: 851. https://doi.org/10.3390/catal13050851