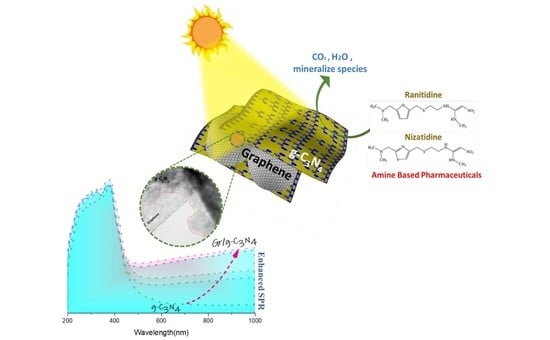
Surface Plasmon Resonance Induced Photocatalysis in 2D/2D Graphene/g-C3N4 Heterostructure for Enhanced Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. SEM, EDX and TEM Analysis
2.3. UV-DRS Analysis
2.4. Photoluminescence Analysis (PL)
2.5. FTIR Analysis
2.6. Photocatalytic Activity Test
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of Gr/g-C3N4 Composite Materials
3.4. Photocatalytic Test of (Gr/g-C3N4)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Al Balushi, B.S.; Al Marzouqi, F.; Al Wahaibi, B.; Kuvarega, A.T.; Al Kindy, S.M.; Kim, Y.; Selvaraj, R. Hydrothermal synthesis of CdS sub-microspheres for photocatalytic degradation of pharmaceuticals. Appl. Surf. Sci. 2018, 457, 559–565. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Lu, L.; Wu, G.; Chen, W. Self-regenerated solar-driven photocatalytic water-splitting by urea derived graphitic carbon nitride with platinum nanoparticles. Chem. Commun. 2012, 48, 8826–8828. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Al-Balushi, N.A.; Kuvarega, A.T.; Karthikeyan, S.; Selvaraj, R. Thermal and hydrothermal synthesis of WO3 nanostructure and its optical and photocatalytic properties for the degradation of Cephalexin and Nizatidine in aqueous solution. Mater. Sci. Eng. B 2021, 264, 114991. [Google Scholar] [CrossRef]
- Al Sarihi, F.T.; Al Marzouqi, F.; Kuvarega, A.T.; Karthikeyan, S.; Selvaraj, R. Easy conversion of BiOCl plates to flowers like structure to enhance the photocatalytic degradation of endocrine disrupting compounds. Mater. Res. Express 2020, 6, 125537. [Google Scholar] [CrossRef]
- Meetani, M.A.; Alaidaros, A.; Hisaindee, S.; Alhamadat, A.; Selvaraj, R.; Al Marzouqi, F.; Rauf, M.A. Photocatalytic degradation of acetaminophen in aqueous solution by Zn-0.2 Cd0. 8S catalyst and visible radiation. Desalination Water Treat. 2019, 138, 270–279. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Kim, Y.; Selvaraj, R. Shifting of the band edge and investigation of charge carrier pathways in the CdS/g-C3N4 heterostructure for enhanced photocatalytic degradation of levofloxacin. New J. Chem. 2019, 43, 9784–9792. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Selvaraj, R.; Kim, Y. Thermal oxidation etching process of g-c3n4nanosheets from their bulk materials and its photocatalytic activity under solar light irradiation. Desalination Water Treat. 2018, 116, 267–276. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Selvaraj, R.; Kim, Y. Rapid photocatalytic degradation of acetaminophen and levofloxacin using g-C3N4 nanosheets under solar light irradiation. Mater. Res. Express 2020, 6, 125538. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L.; Shi, X.; Bai, W. Size-dependent role of gold in g-C3N4/BiOBr/Au system for photocatalytic CO2 reduction and dye degradation. Sol. Energy Mater. Sol. Cells 2016, 157, 406–414. [Google Scholar] [CrossRef]
- Cheng, F.; Yin, H.; Xiang, Q. Low-temperature solid-state preparation of ternary CdS/g-C3N4/CuS nanocomposites for enhanced visible-light photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 391, 432–439. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under LED light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Al Farsi, B.; Kuvarega, A.T.; Al Lawati, H.A.; Al Kindy, S.M.; Kim, Y.; Selvaraj, R. Controlled Microwave-Assisted Synthesis of the 2D-BiOCl/2D-g-C3N4 Heterostructure for the Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. ACS Omega 2019, 4, 4671–4678. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jing, Y.; Ma, X.; Liu, T.; Yang, L.; Liu, B.; Yin, S.; Wei, Y.; Wang, Y. Construction of a well-dispersed Ag/graphene-like gC 3 N 4 photocatalyst and enhanced visible light photocatalytic activity. RSC Adv. 2017, 7, 8688–8693. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Tang, L.; Zeng, G.; Fang, S.; Ouyang, X.; Long, B.; Zhou, Y.; Deng, Y.; Liu, Y.; Wang, J. Self-powered photoelectrochemical aptasensor based on phosphorus doped porous ultrathin g-C3N4 nanosheets enhanced by surface plasmon resonance effect. Biosens. Bioelectron. 2018, 121, 19–26. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Shanker, V. Surface plasmon resonance-induced photocatalysis by Au nanoparticles decorated mesoporous g-C3N4 nanosheets under direct sunlight irradiation. Mater. Res. Bull. 2016, 75, 51–58. [Google Scholar] [CrossRef]
- Wang, H.; Sun, T.; Chang, L.; Nie, P.; Zhang, X.; Zhao, C.; Xue, X. The g-C3N4 nanosheets decorated by plasmonic Au nanoparticles: A heterogeneous electrocatalyst for oxygen evolution reaction enhanced by sunlight illumination. Electrochim. Acta 2019, 303, 110–117. [Google Scholar] [CrossRef]
- Farmani, A.; Mir, A. Graphene sensor based on surface plasmon resonance for optical scanning. IEEE Photonics Technol. Lett. 2019, 31, 643–646. [Google Scholar] [CrossRef]
- Islam, M.; Sultana, J.; Biabanifard, M.; Vafapour, Z.; Nine, M.; Dinovitser, A.; Cordeiro, C.; Ng, B.-H.; Abbott, D. Tunable localized surface plasmon graphene metasurface for multiband superabsorption and terahertz sensing. Carbon 2020, 158, 559–567. [Google Scholar] [CrossRef]
- Ji, M.; Di, J.; Ge, Y.; Xia, J.; Li, H. 2D-2D stacking of graphene-like g-C3N4/Ultrathin Bi4O5Br2 with matched energy band structure towards antibiotic removal. Appl. Surf. Sci. 2017, 413, 372–380. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, L.; Zhu, A.; Liu, Y.; Pan, J. Constructing 2D BiOCl/C3N4 layered composite with large contact surface for visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 897–905. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B Environ. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave synthesis of nanoporous materials. Chemphyschem 2006, 7, 296–319. [Google Scholar] [CrossRef]
- Pu, X.; Zhang, D.; Gao, Y.; Shao, X.; Ding, G.; Li, S.; Zhao, S. One-pot microwave-assisted combustion synthesis of graphene oxide–TiO2 hybrids for photodegradation of methyl orange. J. Alloys Compd. 2013, 551, 382–388. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Zhang, L. Enhanced mineralization of pharmaceuticals by surface oxidation over mesoporous γ-Ti-Al2O3 suspension with ozone. Appl. Catal. B Environ. 2017, 202, 118–126. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Chen, W.; Dong, C.; Li, C. The removal of DON derived from algae cells by Cu-doped TiO2 under sunlight irradiation. Chem. Eng. J. 2015, 280, 588–596. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad, J.; Flora, S. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018, 167, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Xu, J.; Shalom, M.; Piersimoni, F.; Antonietti, M.; Neher, D.; Brenner, T.J. Color-Tunable Photoluminescence and NIR Electroluminescence in Carbon Nitride Thin Films and Light-Emitting Diodes. Adv. Opt. Mater. 2015, 3, 913–917. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Wang, X.; Zhang, X.; Tian, J. Structure engineering of 1T/2H multiphase MoS2 via oxygen incorporation over 2D layered porous g-C3N4 for remarkably enhanced photocatalytic hydrogen evolu-tion. Mater. Today Nano 2022, 18, 100204. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Z.; Sun, D.; Li, Z.; Zhou, W. Hollow semiconductor photocatalysts for solar energy con-version. Adv. Powder Mater. 2022, 1, 100021. [Google Scholar] [CrossRef]
- Liu, X.; Han, X.; Liang, Z.; Xue, Y.; Zhou, Y.; Zhang, X.; Cui, H.; Tian, J. Phosphorous-doped 1T-MoS2 decorated nitrogen-doped g-C3N4 nanosheets for enhanced photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2022, 605, 320–329. [Google Scholar] [CrossRef]
- Wang, C.; Liu, K.; Wang, D.; Wang, G.; Chu, P.K.; Meng, Z.; Wang, X. Hierarchical CuO–ZnO/SiO2 fibrous membranes for efficient removal of congo red and 4-nitrophenol from water. Adv. Fiber Mater. 2022, 4, 1069–1080. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Fu, W.; Sun, Y.; Dai, Y. Core–Sheath CeO2/SiO2 Nanofibers as Nanoreactors for Stabilizing Sinter-Resistant Pt, Enhanced Catalytic Oxidation and Water Remediation. Adv. Fiber Mater. 2022, 4, 1278–1289. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Li, X.; Dong, J.; Zhao, X.; Zhang, Q. Controllable strong and ultralight aramid nanofiber-based aerogel fibers for thermal insulation applications. Adv. Fiber Mater. 2022, 4, 1267–1277. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Liu, Y.; Cai, M.; Zhao, W.; Duan, X. S-scheme MIL-101 (Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr (VI) and tetracycline hydrochloride: Synergistic insights, reaction pathways, and toxicity analysis. Chem. Eng. J. 2023, 455, 140943. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Wang, C.; Lin, W.; Li, S. Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway, mechanism and toxicity assessment. Sep. Purif. Technol. 2023, 304, 122401. [Google Scholar] [CrossRef]
- Andreou, E.K.; Koutsouroubi, E.D.; Vamvasakis, I.; Armatas, G.S. Ni2P-Modified P-Doped Graphitic Carbon Nitride Hetero-Nanostructures for Efficient Photocatalytic Aqueous Cr (VI) Reduction. Catalysts 2023, 13, 437. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Marzouqi, F.; Selvaraj, R. Surface Plasmon Resonance Induced Photocatalysis in 2D/2D Graphene/g-C3N4 Heterostructure for Enhanced Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. Catalysts 2023, 13, 560. https://doi.org/10.3390/catal13030560
Al Marzouqi F, Selvaraj R. Surface Plasmon Resonance Induced Photocatalysis in 2D/2D Graphene/g-C3N4 Heterostructure for Enhanced Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. Catalysts. 2023; 13(3):560. https://doi.org/10.3390/catal13030560
Chicago/Turabian StyleAl Marzouqi, Faisal, and Rengaraj Selvaraj. 2023. "Surface Plasmon Resonance Induced Photocatalysis in 2D/2D Graphene/g-C3N4 Heterostructure for Enhanced Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination" Catalysts 13, no. 3: 560. https://doi.org/10.3390/catal13030560