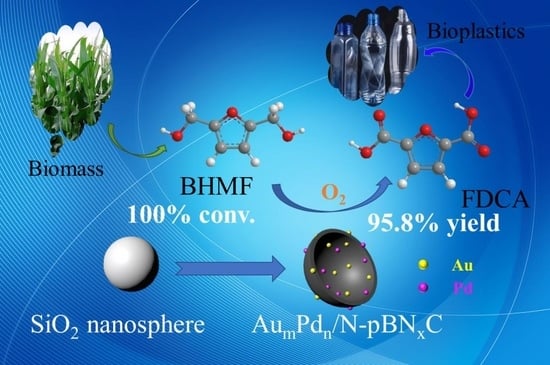
Mechanistic Studies into the Selective Production of 2,5-furandicarboxylic Acid from 2,5-bis(hydroxymethyl)furan Using Au-Pd Bimetallic Catalyst Supported on Nitrated Carbon Material
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the AumPdn/N-BNxC Nanocatalysts
2.2. Catalytic Performance of AumPdn/N-BNxC Nanocatalysts
2.3. Kinetics and Catalytic Mechanism Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of the AumPdn/N-BNxC Nanocatalysts
3.3. Catalyst Characterizations
3.4. BHMF Oxidation Reaction
3.5. Recyclability Test of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhu, B.; Chen, C.L.; Huai, L.Y.; Zhou, Z.Q.; Wang, L.; Zhang, J. 2,5-Bis(hydroxymethyl)furan: A new alternative to HMF for simultaneously electrocatalytic production of FDCA and H2 over CoOOH/Ni electrodes. Appl. Catal. B-Environ. 2021, 297, 120396. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.-V.N.; Chelliapan, S.; Joo, S.-W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Current challenges, innovative insights, and future perspectives. Renew. Sust. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Junaid-Khalil, M.; Arham-Khan, M.; Azad-Alam, M.; Masood, F.; Bazli, L.; Chelliapan, S.; et al. Current trends in hydrogen production, storage and applications in India: A review. Sustain. Energy Technol. 2022, 53, 102677. [Google Scholar] [CrossRef]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2020, 7–8, 100031. [Google Scholar] [CrossRef]
- Mckenna, S.M.; Leimkuehler, S.; Herter, S.; Turner, N.J.; Carnell, A.J. Enzyme cascade reactions: Synthesis of furandicarboxylic acid (FDCA) and carboxylic acids using oxidases in tandem. Green Chem. 2015, 17, 3271–3275. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, Y.L.; Chen, Y.; Wu, J.C.; Cao, Y.; Wei, Y.N.; Huo, P.W. Hierarchical porous bowl-like nitrogen-doped carbon supported bimetallic AuPd nanoparticles as nanoreactors for high efficient catalytic oxidation of HMF to FDCA. J. Catal. 2021, 396, 40–53. [Google Scholar] [CrossRef]
- Siankevich, S.; Savoglidis, G.; Fei, Z.F.; Laurenczy, G.; Alexander, D.T.L.; Yan, N.; Dyson, P.J. A novel platinum nanocatalyst for the oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid under mild conditions. J. Catal. 2014, 315, 67–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent advances in the catalytic synthesis of 2,5-furandicarboxylic acid and its derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Hohl, D.K.; Fleckenstein, P.; Storti, G.; Morbidelli, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701. [Google Scholar] [CrossRef] [Green Version]
- Kucherov, F.A.; Gordeev, E.G.; Kashin, A.S.; Ananikov, V.P. Three-dimensional printing with biomass-derived PEF for carbon-neutral manufacturing. Angew. Chem. Int. Ed. 2017, 56, 15931–15935. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Demet, A.E.; Gimello, O.; Arletti, R.; Tanchoux, N.; Sougrati, M.T.; Stievano, L.; Quignard, F.; Centi, G.; Perathoner, S.; Di-Renzo, F. 5-Hydroxymethylfurfural oxidation to 2,5-furandicarboxylic acid on noble metal-free nanocrystalline mixed oxide catalysts. Catalysts 2022, 12, 814. [Google Scholar] [CrossRef]
- German, D.; Pakrieva, E.; Kolobova, E.; Carabineiro, S.A.C.; Stucchi, M.; Villa, A.; Prati, L.; Bogdanchikova, N.; Cortés-Corberán, V.; Pestryakov, A. Oxidation of 5-hydroxymethylfurfural on supported Ag, Au, Pd and bimetallic Pd-Au catalysts: Effect of the support. Catalysts 2021, 11, 115. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cao, Y.; Yan, C.H.; Liu, W.Y.; Chen, Y.; Guan, W.; Wang, F.; Liu, Y.R.; Huo, P.W. Rationally designed Au-ZrOx interaction for boosting 5-hydroxymethylfurfural oxidation. Chem. Eng. J. 2023, 459, 141644. [Google Scholar] [CrossRef]
- Pichler, C.M.; Al-Shaal, M.G.; Gu, D.; Joshi, H.; Ciptonugroho, W.; Schueth, F. Ruthenium supported on high-surface-area zirconia as an efficient catalyst for the base-free oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. ChemSusChem 2018, 11, 2083–2090. [Google Scholar] [CrossRef]
- Rass, H.A.; Essayem, N.; Besson, M. Selective aqueous phase oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Pt/C catalysts: Influence of the base and effect of bismuth promotion. Green Chem. 2013, 15, 2240–2251. [Google Scholar] [CrossRef]
- Naim, W.; Schade, O.R.; Saraci, E.; Wuest, D.; Kruse, A.; Grunwaldt, J.D. Toward an intensified process of biomass-derived monomers: The influence of 5-(hydroxymethyl)furfural byproducts on the gold-catalyzed synthesis of 2,5-furandicarboxylic acid. ACS Sustain. Chem. Eng. 2020, 8, 11512–11521. [Google Scholar] [CrossRef]
- Megias-Sayago, C.; Chakarova, K.; Penkova, A.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Antonio-Odriozola, J. Understanding the role of the acid sites in 5-hydroxymethylfurfural oxidation to 2,5-furandicarboxylic acid reaction over gold catalysts: Surface investigation on CexZr1-xO2 compounds. ACS Catal. 2018, 8, 11154–11164. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhen, J.; Liu, B.; Lv, K.; Deng, K.J. Selective aerobic oxidation of the biomass-derived precursor 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid under mild conditions over a magnetic palladium nanocatalyst. Green Chem. 2015, 17, 1308–1317. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, K.; Lei, D.; Si, W.; Feng, Y.J.; Lou, L.L.; Liu, S.X. Basicity-tuned hydrotalcite-supported Pd catalysts for aerobic oxidation of 5-hydroxymethyl-2-furfural under mild conditions. ACS Sustain. Chem. Eng. 2016, 4, 4752–4761. [Google Scholar] [CrossRef]
- Jin, M.; Yu, L.; Chen, H.; Ma, X.L.; Cui, K.; Wen, Z.; Ma, Z.W.; Sang, Y.S.; Chen, M.M.; Li, Y.D. Base-free selective conversion of 5-hydroxymethylfurfural to 2,5-furandi-carboxylic acid over a CoOx-CeO2 catalyst. Catal. Today 2021, 367, 2–8. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Lei, D.; Yu, K.; Li, M.-R.; Wang, Y.L.; Liu, T.; Liu, P.K.; Lou, L.L.; Wang, G.C.; Liu, S.X. Facet effect of single-crystalline Pd nanocrystals for aerobic oxidation of 5-hydroxymethyl-2-furfural. ACS Catal. 2017, 7, 421–432. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, C.; Chen, J.S.; Deng, W.P.; Zhang, Q.H.; Yang, Y.H.; Wang, Y. Base-free aerobic oxidation of 5-hydroxymethyl-furfural to 2,5-furandicarboxylic acid in water catalyzed by functionalized carbon nanotube-supported Au-Pd alloy nanoparticles. ACS Catal. 2014, 4, 2175–2185. [Google Scholar] [CrossRef]
- Wei, Y.N.; Zhang, Y.L.; Chen, Y.; Wang, F.; Cao, Y.; Guan, W.; Li, X. Crystal faces-tailored oxygen vacancy in Au/CeO2 catalysts for efficient oxidation of HMF to FDCA. ChemSusChem 2022, 15, e202101983. [Google Scholar] [CrossRef]
- Lin, G.B.; Lin, W.W.; Wu, J.H.; Zhan, Y.; Okejiri, F.; Weng, M.G.; Fu, J. Oxidation of 5–methoxymethylfurfural to 2, 5-furandicarboxylic acid over Ru/hydroxyapatite catalyst in water. Chem. Eng. Sci. 2022, 249, 117343. [Google Scholar] [CrossRef]
- Guan, W.; Chen, C.; Li, B.; Chen, Y.; Wei, Y.N.; Cao, Y.; Wang, F.; Yan, Y.S.; Liu, B.; Zhang, Y.L. Pickering high internal phase emulsions templated CoOx-HPC loading bimetallic AuPd nanoparticles for catalytic oxidation of 5-hydroxymethylfurfural to 2, 5-furan dicarboxylic. ChemistrySelect 2022, 7, e202104058. [Google Scholar] [CrossRef]
- Antonietti, M.; Lopez-Salas, N.; Primo, A. Adjusting the structure and electronic properties of carbons for metal-free carbocatalysis of organic transformations. Adv. Mat. 2019, 31, 1805719. [Google Scholar] [CrossRef]
- Hu, X.W.; Fan, M.Y.; Zhu, Y.Y.; Zhu, Q.; Song, Q.; Dong, Z.P. Biomass-derived phosphorus-doped carbon materials as efficient metal-free catalysts for selective aerobic oxidation of alcohols. Green Chem. 2019, 21, 5274–5283. [Google Scholar] [CrossRef]
- Guo, Z.W.; Zheng, J.; Li, B.R.; Da, Z.L.; Meng, M.J. Fabrication of mixed matrix membranes blending with the TiO2/Bi3O4Cl 2D/2D heterojunction for photocatalytic degradation of tetracycline. Appl. Surf. Sci. 2022, 574, 151549. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Zielinski, M.; Pietrowski, M.; Heyte, S.; Dumeignil, F.; Rossi, L.M.; Wojcieszak, R. Influence of support basic sites in green oxidation of biobased substrates using Au-promoted catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16332–16340. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, Y.L.; Yan, C.H.; Chen, Y.; Wei, Y.N.; Cao, Y.; Wang, F.; Huo, P.W. Base-free aerobic oxidation of furfuralcohols and furfurals to furancarboxylic acids over nitrogen-doped carbon-supported AuPd bowl-like catalyst. ChemSusChem 2022, 15, e202201041. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Cao, D.P.; Huang, L.; Shui, J.L.; Wang, M.; Dai, L.M. Nitrogen-doped holey graphitic carbon from 2D covalent organic polymers for oxygen reduction. Adv. Mater. 2014, 26, 3315. [Google Scholar] [CrossRef]
- Wang, H.B.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, M.; Li, Z.H. CoOx-MC (MC = Mesoporous Carbon) for highly efficient oxidation of 5-hydroxymethylfurfural (5-HMF) to 2,5-furandicarboxylic acid (FDCA). ACS Sustain. Chem. Eng. 2020, 8, 4801–4808. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Campisi, S.; Veith, G.M.; Prati, L. Pd-modified Au on carbon as an effective and durable catalyst for the direct oxidation of HMF to 2,5-furandicarboxylic acid. ChemSusChem 2013, 6, 609–612. [Google Scholar] [CrossRef]
- Lee, K.-U.; Byun, J.Y.; Shin, H.-J.; Kim, S.H. Nanoporous gold-palladium: A binary alloy with high catalytic activity for the electro-oxidation of ethanol. J. Alloys Compd. 2020, 842, 155847. [Google Scholar] [CrossRef]
- Yan, W.; Tang, Z.H.; Wang, L.K.; Wang, Q.N.; Yang, H.Y.; Chen, S.W. PdAu alloyed clusters supported by carbon nanosheets as efficient electrocatalysts for oxygen reduction. Int. J. Hydrogen Energy 2017, 42, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tong, Z.K.; Zhu, S.; Deng, Q.; Chen, S.X.; Wang, J.; Zeng, Z.L.; Zhang, Y.L.; Zou, J.J.; Deng, S.J. Water-mediated hydrogen spillover accelerates hydrogenative ring-rearrangement of furfurals to cyclic compounds. J. Catal. 2022, 405, 363–372. [Google Scholar] [CrossRef]
- Yusuf, M.; Beg, M.; Ubaidullah, M.; Shaikh, S.F.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Kinetic studies for DRM over high-performance Ni–W/Al2O3–MgO catalyst. Int. J. Hydrogen Energy 2022, 47, 42150–42159. [Google Scholar] [CrossRef]









| Samples | Surface Area a (m2g−1) | Average Pore a (nm) | Pore Volume a (cm3g−1) | Nitrogen Content b (%) |
|---|---|---|---|---|
| BN1C | 53.9 | 14.5 | 0.20 | 4.0 |
| BN2C | 35.1 | 13.6 | 0.12 | 7.2 |
| BN3C | 119 | 5.3 | 0.16 | 9.0 |
| N-BN1C | 9.4 | 24 | 0.06 | 9.7 |
| N-BN2C | 13.2 | 24.3 | 0.08 | 10.1 |
| N-BN3C | 11.4 | 16.6 | 0.05 | 11.6 |
| Sample | BHMF Conversion (%) | HMF Yield (%) | HMFCA Yield (%) | FFCA Yield (%) | FDCA Yield (%) | Pd Content (wt%) | Au Content (wt%) |
|---|---|---|---|---|---|---|---|
| Au/N-BN2C | 100 | 0 | 15.9 | 3.6 | 43.9 | 0 | 1.58 |
| Pd/N-BN2C | 100 | 0 | 40.6 | 5.8 | 8.7 | 1.67 | 0 |
| Au2Pd1/N-BN2C | 100 | 0 | 5.5 | 0 | 78.9 | 0.38 | 1.16 |
| Au1Pd1/N-BN2C | 100 | 0 | 0 | 0 | 95.8 | 0.63 | 0.76 |
| Au3Pd1/N-BN2C | 100 | 0 | 25.7 | 0 | 49.6 | 0.26 | 1.35 |
| Au1Pd1/N-BN1C | 100 | 0 | 10.5 | 3.8 | 38.9 | 0.58 | 0.81 |
| Au1Pd1/N-BN3C | 100 | 0 | 15.6 | 4.3 | 50.6 | 0.55 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Y.; Guan, W.; Cao, Y.; Wang, F.; Zhang, Y. Mechanistic Studies into the Selective Production of 2,5-furandicarboxylic Acid from 2,5-bis(hydroxymethyl)furan Using Au-Pd Bimetallic Catalyst Supported on Nitrated Carbon Material. Catalysts 2023, 13, 435. https://doi.org/10.3390/catal13020435
Liu Y, Chen Y, Guan W, Cao Y, Wang F, Zhang Y. Mechanistic Studies into the Selective Production of 2,5-furandicarboxylic Acid from 2,5-bis(hydroxymethyl)furan Using Au-Pd Bimetallic Catalyst Supported on Nitrated Carbon Material. Catalysts. 2023; 13(2):435. https://doi.org/10.3390/catal13020435
Chicago/Turabian StyleLiu, Yiran, Yao Chen, Wen Guan, Yu Cao, Fang Wang, and Yunlei Zhang. 2023. "Mechanistic Studies into the Selective Production of 2,5-furandicarboxylic Acid from 2,5-bis(hydroxymethyl)furan Using Au-Pd Bimetallic Catalyst Supported on Nitrated Carbon Material" Catalysts 13, no. 2: 435. https://doi.org/10.3390/catal13020435