Novel In Vitro Multienzyme Cascade for Efficient Synthesis of d-Tagatose from Sucrose
Abstract
:1. Introduction
2. Results and Discussion
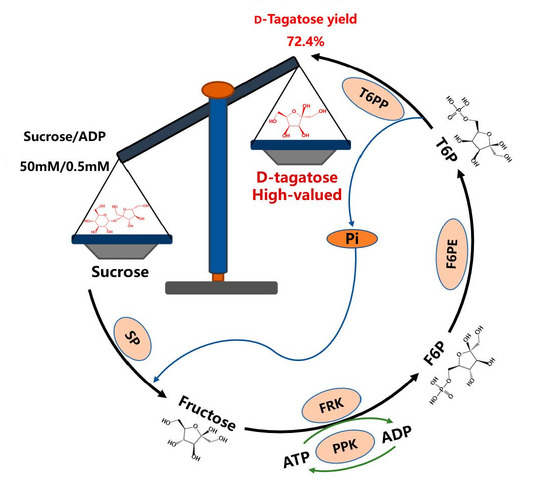
2.1. Design of Multienzyme Cascade Route for d-Tagatose Synthesis from Sucrose
2.2. Feasibility Verification of MCTS
2.3. Construction of ATP Regeneration System
2.4. Optimization of MCTS Route
2.4.1. Enzyme Dosage
2.4.2. Reaction Temperature and pH
2.4.3. Metal Ions
2.4.4. Additional ADP Concentration
2.5. Reaction Time Course
2.6. d-Tagatose Synthesis at Elevated Concentration of Sucrose
3. Materials and Methods
3.1. Genes, Plasmid, and Bacterial Strains
3.2. Chemicals
3.3. Expression and Purification of Enzymes
3.4. Determination of Sucrose, d-Fructose, d-Tagatose, and ATP
3.5. Activity Assay
3.6. Optimization Reaction Conditions for d-Tagatose Synthesis
3.7. Reaction Time Course
3.8. D-Tagatose Production at an Elevated Sucrose Concentration of 50 mM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Levin, G.V.; Donner, T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes. Obes. Metab. 2008, 10, 109–134. [Google Scholar] [CrossRef]
- Oh, D.-K. Tagatose: Properties, applications, and biotechnological processes. Appl. Microbiol. Biot. 2007, 76, 1–8. [Google Scholar] [CrossRef]
- Buemann, B.; Toubro, S.; Raben, A.; Blundell, J.; Astrup, A. The acute effect of d-tagatose on food intake in human subjects. Br. J. Nutr. 2000, 84, 227–231. [Google Scholar] [CrossRef]
- Mooradian, A.D. In search for an alternative to sugar to reduce obesity. Int. J. Vitam. Nutr. Res. 2019, 89, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Levin, G.V. Removal and prevention of dental plaque with d-tagatose. Int. J. Cosmet. Sci. 2010, 24, 225–234. [Google Scholar] [CrossRef]
- Laerke, H.N.; Jensen, B.B.; Højsgaard, S. In vitro fermentation pattern of d-tagatose is affected by adaptation of the microbiota from the gastrointestinal tract of pigs. J. Nutr. 2000, 130, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. d-Tagatose is a promising sweetener to control glycaemia: A new functional food. Biomed. Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef] [PubMed]
- Jayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of d-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Oroskar, A.R.; Kulkarni, O.M.; House, D.W. Tagatose Production Using Simulated Moving Bed Separation. U.S. Patent 8,802,843, 12 August 2014. [Google Scholar]
- Zhan, Y.; Xu, Z.; Li, S.; Liu, X.; Xu, L.; Feng, X.; Xu, H. Coexpression of β-d-galactosidase and l-arabinose isomerase in the production of d-tagatose: A functional sweetener. J. Agric. Food Chem. 2014, 62, 2412–2417. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, R.; Wang, Q.; Hu, M.; Li, Z.; Xu, M.; Yang, T.; Zhang, R.; Rao, Z. Production of d-tagatose by whole-cell conversion of recombinant Bacillus subtilis in the absence of antibiotics. Biology 2021, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.S.; Singh, R.; Kang, Y.C.; Zhao, H.; Lee, J.-K. Cloning and characterization of a galactitol 2-dehydrogenase from Rhizobium legumenosarum and its application in d-tagatose production. Enzym. Microb. Technol. 2014, 58–59, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Z.; Zhang, W.; Guang, C.; Mu, W. Characterization of a d-tagatose 3-epimerase from Caballeronia fortuita and its application in rare sugar production. Int. J. Biol. Macromol. 2019, 138, 536–545. [Google Scholar] [CrossRef]
- Papageorgiou, A.C. Structural characterization of multienzyme assemblies: An overview. Methods Mol. Biol. 2022, 2487, 51–72. [Google Scholar] [PubMed]
- Sun, Z.; Zhao, Q.; Haag, R.; Wu, C. Responsive emulsions for sequential multienzyme cascades. Angew. Chem. Int. Ed. Eng. 2021, 60, 8410–8414. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hong, S.-H.; Kim, K.-R.; Oh, D.-K. High-yield production of pure tagatose from fructose by a three-step enzymatic cascade reaction. Biotechnol. Lett. 2017, 39, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, J.; Zhang, T.; Chen, J.; Hassanin, H.A.; Jiang, B. Characteristics of a fructose 6-phosphate 4-epimerase from Caldilinea aerophila DSM 14535 and its application for biosynthesis of tagatose. Enzym. Microb. Technol. 2020, 139, 109594. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, T.; Jiang, B.; Mu, W.; Chen, J.; Hassanin, H.A. Dictyoglomus turgidum DSM 6724 α-Glucan phosphorylase: Characterization and its application in multi-enzyme cascade reaction for d-tagatose production. Appl. Biochem. Biotechnol. 2021, 193, 3719–3731. [Google Scholar] [CrossRef]
- Dai, Y.; Li, M.; Jiang, B.; Zhang, T.; Chen, J. Whole-cell biosynthesis of d-tagatose from maltodextrin by engineered Escherichia coli with multi-enzyme co-expression system. Enzym. Microb. Technol. 2021, 145, 109747. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Andexer, J.N.; Richter, M. Emerging enzymes for ATP regeneration in biocatalytic processes. ChemBioChem 2015, 16, 380–386. [Google Scholar] [CrossRef]
- Wu, W.; Bergstrom, D.E.; Davisson, V.J. Chemoenzymatic preparation of nucleoside triphosphates. Curr. Protoc. Nucleic Acid. Chem. 2004, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Motomura, K.; Shinoda, Y.; Urata, M.; Kato, J.; Takiguchi, N.; Ohtake, H.; Hirota, R.; Kuroda, A. Use of an Escherichia coli recombinant producing thermostable polyphosphate kinase as an ATP regenerator to produce fructose 1,6-diphosphate. Appl. Environ. Microbiol. 2007, 73, 5676–5678. [Google Scholar] [CrossRef] [PubMed]
- Aiguo, Z.; Ruiwen, D.; Meizhi, Z. Multi-enzymatic recycling of ATP and NADPH for the synthesis of 5-aminolevulinic acid using a semipermeable reaction system. Biosci. Biotechnol. Biochem. 2019, 83, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Y.; Huang, H.; Jin, X.; Li, J.; Du, G.; Kang, Z. Closed-Loop system driven by ADP phosphorylation from pyrophosphate affords equimolar transformation of ATP to 3′-phosphoadenosine-5′-phosphosulfate. ACS Catal. 2021, 11, 10405–10415. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Wang, C.; Zhang, F.; Xu, X.; Zhu, L. Stability improvement of aerobic granular sludge (AGS) based on Gibbs free energy change (∆G) of sludge-water interface. Water Res. 2023, 240, 120059. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.P.J. Enzymatic regeneration and conservation of A1vTP: Challenges and opportunities. Crit. Rev. Biotechnol. 2021, 41, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Rudi, K.; Goa, I.A.; Saltnes, T.; Sørensen, G.; Angell, I.L.; Eikås, S. Microbial ecological processes in MBBR biofilms for biological phosphorus removal from wastewater. Water Sci. Technol. 2019, 79, 1467–1473. [Google Scholar] [CrossRef]
- Nahálka, J.; Gemeiner, P.; Bucko, M.; Wang, P.G. Bioenergy beads: A tool for regeneration of ATP/NTP in biocatalytic synthesis. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 515–521. [Google Scholar] [CrossRef]
- Petchey, M.R.; Rowlinson, B.; Lloyd, R.C.; Fairlamb, I.J.S.; Grogan, G. Biocatalytic synthesis of moclobemide using the amide bond synthetase mcbA coupled with an ATP recycling system. ACS Catal. 2020, 10, 4659–4663. [Google Scholar] [CrossRef]
- Ogawa, M.; Uyeda, A.; Harada, K.; Sato, Y.; Kato, Y.; Watanabe, H.; Honda, K.; Matsuura, T. Class III polyphosphate kinase 2 enzymes catalyze the pyrophosphorylation of adenosine-5’-monophosphate. ChemBioChem 2019, 20, 2961–2967. [Google Scholar] [CrossRef]
- Arcus, V.L.; Prentice, E.J.; Hobbs, J.K.; Mulholland, A.J.; Van der Kamp, M.W.; Pudney, C.R.; Parker, E.J.; Schipper, L.A. On the temperature dependence of enzyme-catalyzed rates. Biochemistry 2016, 55, 1681–1688. [Google Scholar] [CrossRef]
- Alberty, R.A. Effects of pH in rapid-equilibrium enzyme kinetics. J. Phys. Chem. B 2007, 111, 14064–14068. [Google Scholar] [CrossRef]
- Prejanò, M.; Alberto, M.E.; Russo, N.; Toscano, M.; Marino, T. The effects of the metal ion substitution into the active site of metalloenzymes: A theoretical insight on some selected cases. Catalysts 2020, 10, 1038. [Google Scholar] [CrossRef]
- Hu, C.; Wei, X.; Song, Y. A thermophilic phosphatase from Methanothermobacter marburgensis and its application to in vitro biosynthesis. Enzym. Microb. Technol. 2022, 159, 110067. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Mu, W.; Jiang, B. Efficient biotransformation of d-galactose to d-tagatose by Acidothermus cellulolytics ATCC 43068. J. Food. Biochem. 2011, 35, 1298–1310. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Lyu, X.; Wang, C.; Tong, Y.; Hua, X.; Yang, R. Recycling preparation of high-purity tagatose from galactose using one-pot boronate affinity adsorbent-based adsorption-assisted isomerization and simultaneous purification. Chem. Eng. J. 2022, 446, 137089. [Google Scholar] [CrossRef]
- Gao, H.; Li, M.; Wang, Q.; Liu, T.; Zhang, X.; Yang, T.; Xu, M.; Rao, Z. A high-throughput dual system to screen polyphosphate kinase mutants for efficient ATP regeneration in L-theanine biocatalysis. Biotechnol. Biofuels Bioprod. 2023, 16, 122. [Google Scholar]







| Enzyme Name | NCBI Accession Number | Specific Activity (U·mg−1) |
|---|---|---|
| BaSP | WP_011742626.1 | 84 ± 2 |
| CaFRK | KHD36265.1 | 113 ± 3 |
| CaF6PE | WP_014433578 | (2 ± 0.5) × 10–2 |
| MmT6PP | WP_013296249.1 | (1 ± 0.2) × 10–1 |
| Sucrose (mM) | ADP (mM) | Conversion (%) | Production (g·L−1) | Space–Time Yield (g·L−1 h−1) | S:C [b] |
|---|---|---|---|---|---|
| 50 | 0.1 | 61.7 | 5.6 | 0.23 | 18.5 |
| 50 | 0.25 | 62.8 | 5.7 | 0.24 | 18.5 |
| 50 | 0.5 | 72.4 | 6.5 | 0.27 | 18.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tu, W.; Ni, Y.; Guo, Y.; Han, R. Novel In Vitro Multienzyme Cascade for Efficient Synthesis of d-Tagatose from Sucrose. Catalysts 2023, 13, 1515. https://doi.org/10.3390/catal13121515
Liu S, Tu W, Ni Y, Guo Y, Han R. Novel In Vitro Multienzyme Cascade for Efficient Synthesis of d-Tagatose from Sucrose. Catalysts. 2023; 13(12):1515. https://doi.org/10.3390/catal13121515
Chicago/Turabian StyleLiu, Shuangyu, Wenyu Tu, Ye Ni, Yuanyi Guo, and Ruizhi Han. 2023. "Novel In Vitro Multienzyme Cascade for Efficient Synthesis of d-Tagatose from Sucrose" Catalysts 13, no. 12: 1515. https://doi.org/10.3390/catal13121515