Efficient Synthesis of Nickel-Molybdenum/USY-Zeolite Catalyst for Eliminating Impurities (N, S, and Cl) in the Waste Plastic Pyrolysis Oil: Dispersion Effect of Active Sites by Surfactant-Assisted Melt-Infiltration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity Test
2.2. Structure of the Ni-Mo Catalysts
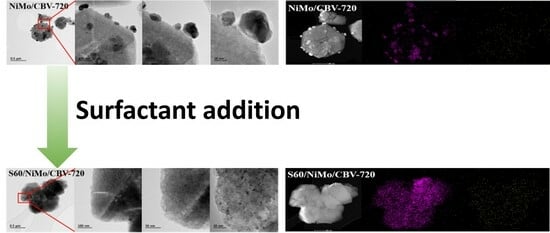
2.3. Highly Dispersed Ni and Mo Particles on a Zeolite Support
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization
3.4. Catalytic Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miandad, R.; Barakat, M.; Rehan, M.; Aburiazaiza, A.; Ismail, I.; Nizami, A. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Al-Salem, S.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Ampah, J.D.; Veza, I.; Atabani, A.; Hoang, A.T.; Nippae, A.; Powoe, M.T.; Afrane, S.; Yusuf, D.A.; Yahuza, I. Investigating the influence of plastic waste oils and acetone blends on diesel engine combustion, pollutants, morphological and size particles: Dehalogenation and catalytic pyrolysis of plastic waste. Energy Convers. Manag. 2023, 291, 117312. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Ampah, J.D.; Soudagar, M.E.M.; Veza, I.; Kingsley, U.; Afrane, S.; Jin, C.; Liu, H.; Elfasakhany, A.; Buyondo, K.A. Effects of hybrid nanoparticle additives in n-butanol/waste plastic oil/diesel blends on combustion, particulate and gaseous emissions from diesel engine evaluated with entropy-weighted PROMETHEE II and TOPSIS: Environmental and health risks of plastic waste. Energy Convers. Manag. 2022, 264, 115758. [Google Scholar]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; van Harmelen, T.; de Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Nabgan, W.; Rashidzadeh, M.; Nabgan, B. The catalytic naphtha reforming process: Hydrodesulfurization, catalysts and zeoforming. Environ. Chem. Lett. 2018, 16, 507–522. [Google Scholar] [CrossRef]
- Al-Salem, S.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Sumarno, S.; Dzawilhijjah, U.; Firmansyah, T.R.; Trisanti, P.N. The effect of ultrasound for impurities removal on spent catalyst from naphtha hydrotreater (NHT) processing unit. In Proceedings of the AIP Conference Proceedings, 2019, Pretoria, South Africa, 18 November 2019. [Google Scholar]
- Rodríguez, E.; Félix, G.; Ancheyta, J.; Trejo, F. Modeling of hydrotreating catalyst deactivation for heavy oil hydrocarbons. Fuel 2018, 225, 118–133. [Google Scholar] [CrossRef]
- Maxwell, I. Zeolite catalysis in hydroprocessing technology. Catal. Today 1987, 1, 385–413. [Google Scholar] [CrossRef]
- Al-Hadhrami, L.M.; Ahmad, A.; Al-Qahtani, A. Performance analysis of heat exchangers of an existing naphtha hydrotreating plant: A case study. Appl. Therm. Eng. 2010, 30, 1029–1033. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Deák, G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feed stocks. Polym. Degrad. Stab. 2006, 91, 517–526. [Google Scholar] [CrossRef]
- Dickerson, T.; Soria, J. Catalytic fast pyrolysis: A review. Energies 2013, 6, 514–538. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Sadighi, S.; Mohaddecy, S.R.S.; Ghabouli, O.; Bahmani, M. Revamp of Naphtha Hydrotreating Process in An I Rani An Refi Nery. Pet. Coal 2009, 51, 45–50. [Google Scholar]
- Bose, D. Design parameters for a hydro desulfurization (HDS) unit for petroleum naphtha at 3500 barrels per day. World Sci. News 2015, 9, 88–100. [Google Scholar]
- Yang, R.; Li, X.; Wu, J.; Zhang, X.; Zhang, Z. The difference in hydrogenation performance between Ni-in-Al2O3 and Ni-on-Al2O3 for hydrotreating of crude 2-ethylhexanol. Korean J. Chem. Eng. 2010, 27, 55–61. [Google Scholar] [CrossRef]
- Kim, D.-W.; Lee, D.-K.; Ihm, S.-K. Preparation of mo nitride catalysts and their applications to the hydrotreating of indole and benzothiophene. Korean J. Chem. Eng. 2002, 19, 587–592. [Google Scholar] [CrossRef]
- Chen, J.; De Crisci, A.G.; Xing, T. Review on catalysis related research at CanmetENERGY. Can. J. Chem. Eng. 2016, 94, 7–19. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Y. Effect of sulfidation process on catalytic performance over unsupported Ni-Mo-W hydrotreating catalysts. Korean J. Chem. Eng. 2017, 34, 1004–1012. [Google Scholar] [CrossRef]
- Jabbarnezhad, P.; Haghighi, M.; Taghavinezhad, P. Synthesis and physicochemical characterization of ZrO2-doped NiMo/Al2O3 nanocatalyst via precipitation and sequential impregnation methods used in hydrodesulfurization of thiophene. Korean J. Chem. Eng. 2015, 32, 1258–1266. [Google Scholar] [CrossRef]
- Park, Y.C.; Rhee, H.-K. The role of nickel in pyridine hydrodenitrogenation over NiMo/Al2O3. Korean J. Chem. Eng. 1998, 15, 411–416. [Google Scholar] [CrossRef]
- Li, D.; Nishijima, A.; Morris, D. Zeolite-supported Ni and Mo catalysts for hydrotreatments: I. Catalytic activity and spectroscopy. J. Catal. 1999, 182, 339–348. [Google Scholar] [CrossRef]
- Li, D.; Nishijima, A.; Morris, D.; Guthrie, G. Activity and structure of hydrotreating Ni, Mo, and Ni–Mo sulfide catalysts supported on γ-Al2O3–USY Zeolite. J. Catal. 1999, 188, 111–124. [Google Scholar] [CrossRef]
- Huang, W.; Wei, Q.; Zhou, Y.; Liu, X.; Liu, M.; Zhang, P.; Xu, Z.; Yu, Z.; Wang, X.; Liu, H. Hydrotreating of diesel fuel over in-situ nickel modified Y zeolite supported Ni-Mo-S catalyst. Catal. Today 2023, 407, 135–145. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, M.; Zhou, Y.; Wei, Q.; Zhang, Q.; Ding, S.; Zhang, Y.; Yu, T.; You, Q. 4, 6-Dimethyldibenzothiophene hydrodesulfurization on nickel-modified USY-supported NiMoS catalysts: Effects of modification method. Energy Fuels 2017, 31, 7445–7455. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Eggenhuisen, T.M. Melt infiltration: An emerging technique for the preparation of novel functional nanostructured materials. Adv. Mater. 2013, 25, 6672–6690. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, preparation and properties of carbon fiber reinforced ultra-high temperature ceramic composites for aerospace applications: A review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Cho, E.H.; Park, Y.-K.; Park, K.Y.; Song, D.; Koo, K.Y.; Jung, U.; Yoon, W.R.; Ko, C.H. Simultaneous impregnation of Ni and an additive via one-step melt-infiltration: Effect of alkaline-earth metal (Ca, Mg, Sr, and Ba) addition on Ni/γ-Al2O3 for CO2 methanation. Chem. Eng. J. 2022, 428, 131393. [Google Scholar] [CrossRef]
- Cho, E.H.; Kim, M.J.; Yoon, B.S.; Kim, Y.J.; Song, D.; Koo, K.Y.; Jung, U.; Jeon, S.-G.; Park, Y.-K.; Ko, C.H. Enhancement in nickel-silica interface generation by surfactant-assisted melt-infiltration: Surfactant selection and application in CO2 hydrogenation. Chem. Eng. J. 2022, 437, 135166. [Google Scholar]
- Cho, E.H.; Jeon, N.; Yoon, B.S.; Kim, S.; Yun, Y.; Ko, C.H. Magnesium-promoted Ni/USY catalysts prepared via surfactant-assisted melt infiltration for ammonia decomposition. Appl. Surf. Sci. 2023, 608, 155244. [Google Scholar] [CrossRef]
- Yoon, B.S.; Kim, K.-J.; Cho, E.H.; Park, H.-R.; Roh, H.-S.; Ko, C.H. Enhanced Fe–Cr dispersion on mesoporous silica support using surfactant-assisted melt-infiltration for the water-gas shift reaction in waste-to-hydrogen processes. Int. J. Hydrogen Energy 2023, 48, 24894–24903. [Google Scholar] [CrossRef]
- Altamirano, E.; de los Reyes, J.A.; Murrieta, F.; Vrinat, M. Hydrodesulfurization of 4, 6-dimethyldibenzothiophene over Co(Ni) MoS2 catalysts supported on alumina: Effect of gallium as an additive. Catal. Today 2008, 133, 292–298. [Google Scholar] [CrossRef]
- Hein, J.; Gutiérrez, O.Y.; Albersberger, S.; Han, J.; Jentys, A.; Lercher, J.A. Towards understanding structure–activity relationships of Ni–Mo–W sulfide hydrotreating catalysts. ChemCatChem 2017, 9, 629–641. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Ni/Al2O3 catalysts for CO methanation: Effect of Al2O3 supports calcined at different temperatures. J. Energy Chem. 2013, 22, 919–927. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, Y.; Jentys, A.; Lercher, J.A. Understanding the impact of aluminum oxide binder on Ni/HZSM-5 for phenol hydrodeoxygenation. Appl. Catal. B Environ. 2013, 132, 282–292. [Google Scholar] [CrossRef]
- Arnoldy, P.; De Jonge, J.; Moulijn, J. Temperature-programed reduction of molybdenum (VI) oxide and molybdenum (IV) oxide. J. Phys. Chem. 1985, 89, 4517–4526. [Google Scholar] [CrossRef]
- Burch, R.; Collins, A. Temperature-programmed reduction of Ni/Mo hydrotreating catalysts. Appl. Catal. 1985, 18, 389–400. [Google Scholar] [CrossRef]





| BET Surface Area a [A, m2 g−1] | Total Pore Volume b [Vp, cm3 g−1] | Average Pore Diameter c [dp, nm] | |
|---|---|---|---|
| Pure CBV 720 | 930 | 0.56 | 2.4 |
| Ni/CBV 720 | 950 | 0.57 | 2.4 |
| NiMo/CBV 720 | 630 | 0.44 | 2.8 |
| S60/NiMo/CBV 720 | 530 | 0.36 | 2.7 |
| Pure WSY-60H | 820 | 0.55 | 2.6 |
| Ni/WSY-60H | 860 | 0.60 | 2.8 |
| NiMo/WSY-60H | 470 | 0.34 | 2.9 |
| S60/NiMo/WSY-60H | 530 | 0.36 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.H.; Kim, K.-D.; Yoon, B.S.; Cho, E.; Yu, Y.J.; Phan, T.N.; Jeon, S.-G.; Ko, C.H. Efficient Synthesis of Nickel-Molybdenum/USY-Zeolite Catalyst for Eliminating Impurities (N, S, and Cl) in the Waste Plastic Pyrolysis Oil: Dispersion Effect of Active Sites by Surfactant-Assisted Melt-Infiltration. Catalysts 2023, 13, 1476. https://doi.org/10.3390/catal13121476
Cho EH, Kim K-D, Yoon BS, Cho E, Yu YJ, Phan TN, Jeon S-G, Ko CH. Efficient Synthesis of Nickel-Molybdenum/USY-Zeolite Catalyst for Eliminating Impurities (N, S, and Cl) in the Waste Plastic Pyrolysis Oil: Dispersion Effect of Active Sites by Surfactant-Assisted Melt-Infiltration. Catalysts. 2023; 13(12):1476. https://doi.org/10.3390/catal13121476
Chicago/Turabian StyleCho, Eui Hyun, Ki-Duk Kim, Byung Sun Yoon, Eunkyung Cho, Yeon Jeong Yu, Tuan Ngoc Phan, Sang-Goo Jeon, and Chang Hyun Ko. 2023. "Efficient Synthesis of Nickel-Molybdenum/USY-Zeolite Catalyst for Eliminating Impurities (N, S, and Cl) in the Waste Plastic Pyrolysis Oil: Dispersion Effect of Active Sites by Surfactant-Assisted Melt-Infiltration" Catalysts 13, no. 12: 1476. https://doi.org/10.3390/catal13121476