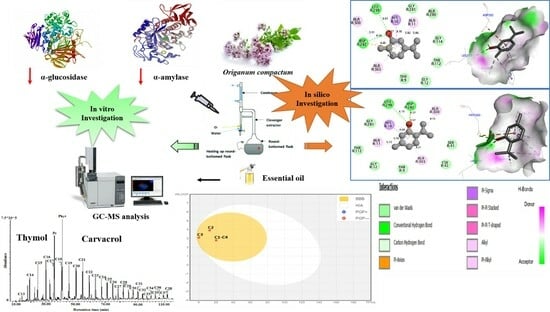
GC/MS Profiling, In Vitro Antidiabetic Efficacy of Origanum compactum Benth. Essential Oil and In Silico Molecular Docking of Its Major Bioactive Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
2.2. Antidiabetic Activity
2.3. ADME and Toxicity Prediction
2.4. Molecular Docking
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and EO Extraction
3.3. GC-MS Analysis
3.4. In Vitro Antidiabetic Assay
3.4.1. α-Amylase Inhibition Assay
3.4.2. α-Glucosidase Inhibition Assay
3.5. ADMET Prediction
3.6. Molecular Docking Study
3.7. Strengths and Weaknesses of Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balwan, W.K.; Saba, N.; Zargar, J.I. Burden of Diabetes and Role of Medicinal Plants in Its Treatment. Saudi J. Med. Pharm. Sci. 2022, 8, 355–361. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Nabrdalik, K.; Kwiendacz, H.; Moos, J.; Moos, Ł.; Kulpa, J.; Brzoza, Z.; Stompór, T.; Gumprecht, J.; Lip, G.Y. Diabetic Peripheral Neuropathy Is Associated with Diabetic Kidney Disease and Cardiovascular Disease–The Silesia Diabetes-Heart Project. Curr. Probl. Cardiol. 2023, 48, 101726. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.A.; Singh, R.; Bhuyan, J.R.; Prusty, B.; Hotta, S.K.; Anusha, V.L.; Kumar, K.R.; Nagaraju, G.V. A detailed overview on current developments in treatment strategies for diabetes mellitus management. Eur. Chem. Bull. 2023, 12, 2069–2083. [Google Scholar]
- Garcia-Molina, L.; Lewis-Mikhael, A.-M.; Riquelme-Gallego, B.; Cano-Ibanez, N.; Oliveras-Lopez, M.-J.; Bueno-Cavanillas, A. Improving Type 2 Diabetes Mellitus Glycaemic Control through Lifestyle Modification Implementing Diet Intervention: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2020, 59, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Y.; Xu, X.-Y.; Gan, R.-Y.; Sun, Q.-C.; Meng, J.-M.; Shang, A.; Mao, Q.-Q.; Li, H.-B. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods 2019, 8, 440. [Google Scholar] [CrossRef]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S. Bioactive Compounds Effective against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.-H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK Signaling Pathway by Natural Products for Treatment of Diabetes Mellitus and Its Complications. J. Cell. Physiol. 2019, 234, 17212–17231. [Google Scholar] [CrossRef]
- Khan, S.J.; Afroz, S.; Khan, R.A. Antihyperlipidemic and Anti-Hyperglycemic Effects of Cymbopogon Jwarancusa in High-Fat High-Sugar Diet Model. Pak. J. Pharm. Sci. 2018, 31, 1341–1345. [Google Scholar]
- Qiao, W.; Zhao, C.; Qin, N.; Zhai, H.Y.; Duan, H.Q. Identification of Trans-Tiliroside as Active Principle with Anti-Hyperglycemic, Anti-Hyperlipidemic and Antioxidant Effects from Potentilla Chinesis. J. Ethnopharmacol. 2011, 135, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Essential Oil-Bearing Plants from Balkan Peninsula: Promising Sources for New Drug Candidates for the Prevention and Treatment of Diabetes Mellitus and Dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations When Using Alpha-Glucosidase Inhibitors in the Treatment of Type 2 Diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Jaradat, N.; Shraim, N.; Hawash, M.; Issa, L.; Shakhsher, M.; Nawahda, N.; Hanbali, A.; Barahmeh, N.; Taha, B. Assessment of Anticancer, Antimicrobial, Antidiabetic, Anti-Obesity and Antioxidant Activity of Ocimum Basilicum Seeds Essential Oil from Palestine. BMC Complement. Med. Ther. 2023, 23, 221. [Google Scholar] [CrossRef]
- Ibrahim, F.M.; Fouad, R.; EL-Hallouty, S.; Hendawy, S.F.; Omer, E.A.; Mohammed, R.S. Egyptian Myrtus communis L. Essential Oil Potential Role as Invitro Antioxidant, Cytotoxic and α-Amylase Inhibitor. Egypt. J. Chem. 2021, 64, 3005–3017. [Google Scholar] [CrossRef]
- Xu, F.; Gu, D.; Wang, M.; Zhu, L.; Chu, T.; Cui, Y.; Tian, J.; Wang, Y.; Yang, Y. Screening of the Potential α-Amylase Inhibitor in Essential Oil from Cedrus Deodara Cones. Ind. Crops Prod. 2017, 103, 251–256. [Google Scholar] [CrossRef]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Rosemary (Rosmarinus officinalis) Essential Oil Components Exhibit Anti-Hyperglycemic, Anti-Hyperlipidemic and Antioxidant Effects in Experimental Diabetes. Pathophysiology 2017, 24, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Cagliero, C.; Marengo, A.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Bio-Guided Fractionation Driven by in Vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity. Plants 2020, 9, 1242. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Legssyer, A.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory Effect of Roasted/Unroasted Argania Spinosa Seeds Oil on α-Glucosidase, α-Amylase and Intestinal Glucose Absorption Activities. S. Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- Bouyahya, A.; Zengin, G.; Belmehdi, O.; Bourais, I.; Chamkhi, I.; Taha, D.; Benali, T.; Dakka, N.; Bakri, Y. Origanum Compactum Benth., from Traditional Use to Biotechnological Applications. J. Food Biochem. 2020, 44, e13251. [Google Scholar] [CrossRef]
- Btissam, R.; Rajae, R.; Amina, A.; Brigitte, V.; Mohamed, N. In Vitro Study of Anti-Glycation and Radical Scavenging Activities of the Essential Oils of Three Plants from Morocco: Origanum compactum, Rosmarinus officinalis and Pelargonium asperum. Pharmacogn. J. 2015, 7, 124–135. [Google Scholar] [CrossRef]
- Bouhdid, S.; Skali, S.N.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial and Antioxidant Activities of Origanum compactum Essential Oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar]
- El Abdali, Y.; Mahraz, A.M.; Beniaich, G.; Mssillou, I.; Chebaibi, M.; Bin Jardan, Y.A.; Lahkimi, A.; Nafidi, H.-A.; Aboul-Soud, M.A.; Bourhia, M. Essential Oils of Origanum Compactum Benth: Chemical Characterization, in Vitro, in Silico, Antioxidant, and Antibacterial Activities. Open Chem. 2023, 21, 20220282. [Google Scholar] [CrossRef]
- Jeddi, M.; Fikri-Benbrahim, K.; El Hachlafi, N.; Benkhaira, N.; Aboussemdai, A.; Ouaritini, Z.B. Chemical Composition of Thymus Vulgaris, Origanum Compactum and Vetiveria Zizanoides Essential Oils and Their Antibacterial and Antioxidant Activities. Trop. J. Nat. Prod. Res. 2023, 7, 2244–2250. [Google Scholar]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential Oils of Origanum Compactum Increase Membrane Permeability, Disturb Cell Membrane Integrity, and Suppress Quorum-Sensing Phenotype in Bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Laghmouchi, Y.; Belmehdi, O.; Senhaji, N.S.; Abrini, J. Chemical Composition and Antibacterial Activity of Origanum Compactum Benth. Essential Oils from Different Areas at Northern Morocco. S. Afr. J. Bot. 2018, 115, 120–125. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Zouine, N.; Fadil, M.; Jeddi, M.; Jeddi, S.; Flouchi, R.; Koraichi, S.I.; Fikri-Benbrahim, K. Exploration of Novel Antibacterial and Anti-Adhesive Formulations from Three Chemically Characterized Essential Oils: Optimization Using Experimental Design Methodology. Sci. Afr. 2023, 22, e01927. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Mrabti, H.N.; Al-Mijalli, S.H.; Jeddi, M.; Abdallah, E.M.; Benkhaira, N.; Hadni, H.; Assaggaf, H.; Qasem, A.; Goh, K.W. Antioxidant, Volatile Compounds; Antimicrobial, Anti-Inflammatory, and Dermatoprotective Properties of Cedrus Atlantica (Endl.) Manetti Ex Carriere Essential Oil: In Vitro and In Silico Investigations. Molecules 2023, 28, 5913. [Google Scholar] [CrossRef]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual Screening of Natural Products against Type II Transmembrane Serine Protease (TMPRSS2), the Priming Agent of Coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef]
- Muhammad, I.; Rahman, N.; Niaz, S.; Basharat, Z.; Rastrelli, L.; Jayanthi, S.; Efferth, T.; Khan, H. Screening of Potent Phytochemical Inhibitors against SARS-CoV-2 Protease and Its Two Asian Mutants. Comput. Biol. Med. 2021, 133, 104362. [Google Scholar] [CrossRef] [PubMed]
- Basharat, Z.; Khoso, A. Yaws (Endemic Treponematoses) Drug Discovery from Phytochemicals: An Informatics Protocol for Drug Target Identification to Phytochemical Inhibitor Screening and Validation. In Neglected Tropical Diseases and Phytochemicals in Drug Discovery; Wiley: Hoboken, NJ, USA, 2021; pp. 397–415. [Google Scholar]
- Basharat, Z.; Akhtar, U.; Khan, K.; Alotaibi, G.; Jalal, K.; Abbas, M.N.; Hayat, A.; Ahmad, D.; Hassan, S.S. Differential Analysis of Orientia Tsutsugamushi Genomes for Therapeutic Target Identification and Possible Intervention through Natural Product Inhibitor Screening. Comput. Biol. Med. 2022, 141, 105165. [Google Scholar] [CrossRef] [PubMed]
- Hayani, M.; Bencheikh, N.; Ailli, A.; Bouhrim, M.; Elbouzidi, A.; Ouassou, H.; Kharchoufa, L.; Baraich, A.; Atbir, A.; Ayyad, F.Z. Quality Control, Phytochemical Profile, and Antibacterial Effect of Origanum Compactum Benth. Essential Oil from Morocco. Int. J. Plant Biol. 2022, 13, 546–560. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Balouiri, M.; Bouhdid, S.; Moja, S.; Chahdi, F.O.; Taleb, M.; Greche, H. Mixture Design of Origanum Compactum, Origanum Majorana and Thymus Serpyllum Essential Oils: Optimization of Their Antibacterial Effect. Ind. Crops Prod. 2016, 89, 1–9. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.-J.; Haroutounian, S.A. Essential Oils of Satureja, Origanum, and Thymus Species: Chemical Composition and Antibacterial Activities against Foodborne Pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical Diversity in Leaf and Stem Essential Oils of Origanum vulgare L. and Their Effects on Microbicidal Activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef]
- Yousif, L.; Belmehdi, O.; Abdelhakim, B.; Skali Senhaji, N.; Abrini, J. Does the Domestication of Origanum Compactum (Benth) Affect Its Chemical Composition and Antibacterial Activity? Flavour Fragr. J. 2021, 36, 264–271. [Google Scholar] [CrossRef]
- Mohammed, E.; Fliou, J.; Riffi, O.; Amechrouq, A.; Ghouati, Y. Comparative Study of the Chemical Composition of the Essential Oil of Origanum Compactum from the Seven Regions of Morocco and Their Antimicrobial Activity. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 42–48. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Al-Mijalli, S.H.; Mrabti, H.N.; Abdnim, R.; Abdallah, E.M.; Jeddi, M.; Bnouham, M.; Lee, L.-H.; Ardianto, C. Phytochemical Analysis and Evaluation of Antimicrobial, Antioxidant, and Antidiabetic Activities of Essential Oils from Moroccan Medicinal Plants: Mentha Suaveolens, Lavandula Stoechas, and Ammi Visnaga. Biomed. Pharmacother. 2023, 164, 114937. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Chebat, A.; Fikri-Benbrahim, K. Ethnopharmacology, Phytochemistry, and Pharmacological Properties of Thymus Satureioides Coss. Evid.-Based Complement. Altern. Med. 2021, 2021, 6673838. [Google Scholar] [CrossRef]
- Aimad, A.; Youness, E.A.; Sanae, R.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Nafidi, H.-A. Chemical Composition and Antifungal, Insecticidal and Repellent Activity of Essential Oils from Origanum Compactum Benth. Used in the Mediterranean Diet. Front. Plant Sci. 2022, 13, 798259. [Google Scholar] [CrossRef]
- Kajaria, D.; Tripathi, J.; Tripathi, Y.B.; Tiwari, S. In-Vitro α Amylase and Glycosidase Inhibitory Effect of Ethanolic Extract of Antiasthmatic Drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed]
- Chipiti, T.; Ibrahim, M.A.; Singh, M.; Islam, M.S. In Vitro α-Amylase and α-Glucosidase Inhibitory Effects and Cytotoxic Activity of Albizia Antunesiana Extracts. Pharmacogn. Mag. 2015, 11, S231. [Google Scholar]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential Oil Composition and Antibacterial Activity of Origanum vulgare Subsp. Glandulosum Desf. at Different Phenological Stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum Vulgare Subspecies (Subsp. Vulgare and Subsp. Hirtum) Essential Oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.-C.; Kim, J.-S. Antioxidant and Antidiabetic Activity of Thymus Quinquecostatus Celak. Ind. Crops Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Benkhaira, N.; El Hachlafi, N.; Jeddi, M.; Abdnim, R.; Bnouham, M.; Koraichi, S.I.; Fikri-Benbrahim, K. Unveiling the Phytochemical Profile, in Vitro Bioactivities Evaluation, in Silico Molecular Docking and ADMET Study of Essential Oil from Clinopodium Nepeta Grown in Middle Atlas of Morocco. Biocatal. Agric. Biotechnol. 2023, 54, 102923. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G. Integrated Analysis of Antimicrobial, Antioxidant, and Phytochemical Properties of Cinnamomum Verum: A Comprehensive In Vitro and In Silico Study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Mrabti, H.N.; El Hachlafi, N.; Al-Mijalli, S.H.; Jeddi, M.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G. Phytochemical Profile, Assessment of Antimicrobial and Antioxidant Properties of Essential Oils of Artemisia herba-alba Asso., and Artemisia dracunculus L.: Experimental and Computational Approaches. J. Mol. Struct. 2023, 1294, 136479. [Google Scholar] [CrossRef]
- El Fadili, M.; Er-rajy, M.; Imtara, H.; Kara, M.; Zarougui, S.; Altwaijry, N.; Al Kamaly, O.; Al Sfouk, A.; Elhallaoui, M. 3D-QSAR, ADME-Tox in Silico Prediction and Molecular Docking Studies for Modeling the Analgesic Activity against Neuropathic Pain of Novel NR2B-Selective NMDA Receptor Antagonists. Processes 2022, 10, 1462. [Google Scholar] [CrossRef]
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-F.; Hsieh, C.-T.; Hsieh, T.-J.; Chang, F.-R.; Wang, C.-K. In Vitro Anti-Diabetic Effect and Chemical Component Analysis of 29 Essential Oils Products. J. Food Drug Anal. 2015, 23, 124–129. [Google Scholar] [CrossRef]
- Benkhaira, N.; Zouine, N.; Fadil, M.; Koraichi, S.I.; Hachlafi, N.E.; Jeddi, M.; Lachkar, M.; Fikri-Benbrahim, K. Application of Mixture Design for the Optimum Antibacterial Action of Chemically-Analyzed Essential Oils and Investigation of the Antiadhesion Ability of Their Optimal Mixtures on 3D Printing Material. Bioprinting 2023, 34, e00299. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; El Hachlafi, N.; Abdallah, E.M.; Jeddi, M.; Assaggaf, H.; Qasem, A.; Alnasser, S.M.; Attar, A.; Naem, M.A.; Lee, L.-H. Exploring the Antibacterial Mechanisms of Chemically Characterized Essential Oils from Leaves and Buds of Syzygium Aromaticum (L.) Merr. et Perry against Staphylococcus Aureus and Pseudomonas Aeruginosa. Ind. Crops Prod. 2023, 205, 117561. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2001; ISBN 0-931710-85-5. [Google Scholar]
- Mrabti, H.N.; Sayah, K.; Jaradat, N.; Kichou, F.; Ed-Dra, A.; Belarj, B.; Cherrah, Y.; Faouzi, M.E.A. Antidiabetic and Protective Effects of the Aqueous Extract of Arbutus unedo L. in Streptozotocin-Nicotinamide-Induced Diabetic Mice. J. Complement. Integr. Med. 2018, 15, 20170165. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. In Vitro Models for Selection of Development Candidatesexperimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug. Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Forli, S. Using AutoDock 4 and AutoDock Vina with AutoDockTools: A Tutorial; The Scripps Research Institute Molecular Graphics Laboratory: La Jolla, CA, USA, 2012; p. 1000. [Google Scholar]
- Jejurikar, B.L.; Rohane, S.H. Drug Designing in Discovery Studio. Asian J. Res. Chem. 2021, 14, 135–138. [Google Scholar]
- Systèmes, D. Free Download: BIOVIA Discovery Studio Visualizer-Dassault Systèmes, 2020.
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef]







| No a | Compounds b | KI c | Molecular Formula | % Relative Peak Area |
|---|---|---|---|---|
| 1 | α-Thujene | 923 | C10H16 | 0.41 |
| 2 | α-Pinene | 979 | C10H16 | 0.17 |
| 3 | p-Cymene | 1026 | C10H16 | 7.12 |
| 4 | α-Phellandrene | 1035 | C10H16 | 0.54 |
| 5 | Linalool | 1098 | C10H18O | 2.64 |
| 6 | α-Terpineol | 1185 | C10H18O | 0.28 |
| 7 | Thymol | 1290 | C10H14O | 54.6 |
| 8 | Carvacrol | 1298 | C10H14O | 23.18 |
| 9 | β-Caryophyllene | 1494 | C15H24 | 3.37 |
| 10 | Caryophyllene oxide | 1573 | C15H24O | 5.34 |
| Total identified % | 97.65 | |||
| Monoterpene hydrocarbons | 8.24 | |||
| Oxygenated monoterpenes | 80.7 | |||
| Sesquiterpene hydrocarbons | 3.37 | |||
| Oxygenated sesquiterpenes | 5.34 | |||
| Samples | IC50 (µg/mL) | |
|---|---|---|
| α-Amylase | α-Glucosidase | |
| O. compactum EO | 150.11 ± 0.12 | 119.84 ± 0.22 |
| Acarbose (control) | 98.24 ± 0.03 | 0.02 |
| Compound Numbers | Physico-Chemical Properties | Lipinski Rules | |||||
|---|---|---|---|---|---|---|---|
| Molecular Weight (g/mol) | Molar Refractive Index | Rotatable Bonds | Log P (Octanol/Water) | H-BA | H-BD | Categorical (Yes/No) | |
| Rule | ≤500 | 40 ≤ MR ≤ 130 | <10 | <5 | ≤10 | <5 | Yes/No |
| C1 | 150.22 | 48.01 | 1 | 2.24 | 1 | 1 | Yes |
| C2 | 220.35 | 68.27 | 0 | 3.15 | 1 | 0 | Yes |
| C3 | 134.22 | 45.99 | 1 | 2.51 | 0 | 0 | Yes |
| C4 | 150.22 | 48.01 | 1 | 2.32 | 1 | 1 | Yes |
| Hepatotoxicity | Absorption | Distribution | Metabolism | Excretion | Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intestinal Absorption | BBB Permeability | CNS Permeability | Substrate | Inhibitor | Total Clearance | AMES Toxicity | Hepatotoxicity | Skin Sensitization | ||||||
| Cytochromes | ||||||||||||||
| 2D6 | 3A4 | 1A2 | 2C19 | 2C9 | 2D6 | 3A4 | ||||||||
| Numeric (% Absorbed) | Numeric (Log BB) | Numeric (Log PS) | Categorical (Yes/No) | Numeric (Log mL/min/kg) | Categorical (Yes/No) | |||||||||
| C1 | 93.712 | 0.381 | −1.438 | No | No | No | No | No | No | No | 0.243 | No | No | Yes |
| C2 | 96.066 | 0.654 | −2.525 | No | No | Yes | Yes | Yes | No | No | 0.905 | No | No | Yes |
| C3 | 95.52 | 0.541 | −1.348 | No | No | Yes | No | No | No | No | 0.239 | No | No | Yes |
| C4 | 93.24 | 0.366 | −1.349 | No | No | Yes | No | No | No | No | 0.259 | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assaggaf, H.; El Hachlafi, N.; El fadili, M.; Elbouzidi, A.; Ouassou, H.; Jeddi, M.; Alnasser, S.M.; Qasem, A.; Attar, A.; AL-Farga, A.; et al. GC/MS Profiling, In Vitro Antidiabetic Efficacy of Origanum compactum Benth. Essential Oil and In Silico Molecular Docking of Its Major Bioactive Compounds. Catalysts 2023, 13, 1429. https://doi.org/10.3390/catal13111429
Assaggaf H, El Hachlafi N, El fadili M, Elbouzidi A, Ouassou H, Jeddi M, Alnasser SM, Qasem A, Attar A, AL-Farga A, et al. GC/MS Profiling, In Vitro Antidiabetic Efficacy of Origanum compactum Benth. Essential Oil and In Silico Molecular Docking of Its Major Bioactive Compounds. Catalysts. 2023; 13(11):1429. https://doi.org/10.3390/catal13111429
Chicago/Turabian StyleAssaggaf, Hamza, Naoufal El Hachlafi, Mohamed El fadili, Amine Elbouzidi, Hayat Ouassou, Mohamed Jeddi, Sulaiman Mohammed Alnasser, Ahmed Qasem, Ammar Attar, Ammar AL-Farga, and et al. 2023. "GC/MS Profiling, In Vitro Antidiabetic Efficacy of Origanum compactum Benth. Essential Oil and In Silico Molecular Docking of Its Major Bioactive Compounds" Catalysts 13, no. 11: 1429. https://doi.org/10.3390/catal13111429