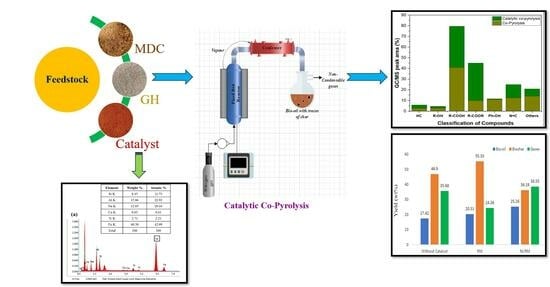
Catalytic Co-Pyrolysis of Mesua ferrea L. De-Oiled Cake and Garlic Husk in the Presence of Red-Mud-Based Catalysts
Abstract
:1. Introduction
2. Results and Discussions
2.1. Physicochemical Characterization of Biomass Feedstock
2.2. Functional Group Analysis of Biomass, Catalyst and Bio-Oil
2.3. Thermal Decomposition of Biomass
2.4. Characterization of Catalyst
2.4.1. Surface Area Analysis by BET
2.4.2. Morphological Analysis of Catalysts by FESEM
2.4.3. Elemental Analysis of Catalyst by Energy Dispersive X-ray Analysis
2.4.4. X-ray Diffraction Pattern of the Synthesized Catalysts
2.5. Thermal Pyrolysis and Pyrolytic Product Distribution
2.6. Catalytic Co-Pyrolysis and Pyrolytic Product Distribution
3. Material and Methods
3.1. Materials
Catalyst Preparation
3.2. Thermal, Co-Pyrolysis and Catalytic Co-Pyrolysis of Biomass
3.3. Biomass, Catalyst, and Pyrolytic Liquid Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V. Adsorptive removal of Zn, Fe, and Pb from Zn dominant simulated industrial wastewater solution using polyvinyl alcohol grafted chitosan variant resins. Chem. Eng. J. 2023, 459, 141563. [Google Scholar] [CrossRef]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. Origin of carbon in aromatic and olefin products derived from HZSM-5 catalyzed co-pyrolysis of cellulose and plastics via isotopic labeling. Appl. Catal. B Environ. 2015, 162, 338–345. [Google Scholar] [CrossRef]
- Reshad, A.S.; Tiwari, P.; Goud, V.V. Thermal and co-pyrolysis of rubber seed cake with waste polystyrene for bio-oil production. J. Anal. Appl. Pyrolysis 2019, 139, 333–343. [Google Scholar] [CrossRef]
- Komandur, J.; Mohanty, K. Chapter 2—Fast pyrolysis of biomass and hydrodeoxygenation of bio-oil for the sustainable production of hydrocarbon biofuels. In Hydrocarbon Biorefinery; Maity, S.K., Gayen, K., Bhowmick, T.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–76. [Google Scholar] [CrossRef]
- Komandur, J.; Vinu, R.; Mohanty, K. Pyrolysis kinetics and pyrolysate composition analysis of Mesua ferrea L.: A non-edible oilseed towards the production of sustainable renewable fuel. Bioresour. Technol. 2022, 351, 126987. [Google Scholar] [CrossRef] [PubMed]
- Agrawalla, A.; Kumar, S.; Singh, R. Pyrolysis of groundnut de-oiled cake and characterization of the liquid product. Bioresour. Technol. 2011, 102, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Naresh Kumar, A.; Chatterjee, S.; Hemalatha, M.; Althuri, A.; Min, B.; Kim, S.-H.; Mohan, S.V. Deoiled algal biomass derived renewable sugars for bioethanol and biopolymer production in biorefinery framework. Bioresour. Technol. 2020, 296, 122315. [Google Scholar] [CrossRef]
- Singh, R.K.; Patil, T.; Sawarkar, A.N. Pyrolysis of garlic husk biomass: Physico-chemical characterization, thermodynamic and kinetic analyses. Bioresour. Technol. Rep. 2020, 12, 100558. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, C.; Xu, J.; Yang, X. Beneficial synergetic effect on gas production during co-pyrolysis of sewage sludge and biomass in a vacuum reactor. Bioresour. Technol. 2015, 183, 255–258. [Google Scholar] [CrossRef]
- Zuo, W.; Jin, B.; Huang, Y.; Sun, Y. Characterization of top phase oil obtained from co-pyrolysis of sewage sludge and poplar sawdust. Environ. Sci. Pollut. Res. 2014, 21, 9717–9726. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Chen, F.; Zhang, Y. Solar pyrolysis of cotton stalks: Combined effects of torrefaction pretreatment and HZSM-5 zeolite on the bio-fuels upgradation. Energy Convers. Manag. 2022, 261, 115640. [Google Scholar] [CrossRef]
- Valizadeh, S.; Ko, C.H.; Lee, J.; Lee, S.H.; Yu, Y.J.; Show, P.L.; Rhee, G.H.; Park, Y.-K. Effect of eggshell- and homo-type Ni/Al2O3 catalysts on the pyrolysis of food waste under CO2 atmosphere. J. Environ. Manag. 2021, 294, 112959. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of pinyon-juniper catalytic pyrolysis oil using red mud-supported nickel catalysts. Appl. Catal. B Environ. 2018, 236, 1–12. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhai, Y.; Li, S.; Liu, X.; Liu, X.; Wang, B.; Liu, Y.; Li, C.; Hu, Y. Catalytic co-pyrolysis of sewage sludge and rice husk over biochar catalyst: Bio-oil upgrading and catalytic mechanism. Waste Manag. 2020, 114, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumar, V.T.; Krishna, B.B.; Bhaskar, T. Characterization of slow pyrolysis products from three different cashew wastes. Bioresour. Technol. 2023, 376, 128859. [Google Scholar] [CrossRef] [PubMed]
- Komandur, J.; Kumar, A.; Para, P.; Mohanty, K. Kinetic Parameters Estimation of Thermal and Co-Pyrolysis of Groundnut De-oiled Cake and Polyethylene Terephthalate (PET) Waste. Energies 2022, 15, 7502. [Google Scholar] [CrossRef]
- Setter, C.; Silva, F.T.M.; Assis, M.R.; Ataíde, C.H.; Trugilho, P.F.; Oliveira, T.J.P. Slow pyrolysis of coffee husk briquettes: Characterization of the solid and liquid fractions. Fuel 2020, 261, 116420. [Google Scholar] [CrossRef]
- Sharma, R.; Sheth, P.N. Multi reaction apparent kinetic scheme for the pyrolysis of large size biomass particles using macro-TGA. Energy 2018, 151, 1007–1017. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Titiloye, J.O. Catalytic pyrolysis of rice husk for bio-oil production. J. Anal. Appl. Pyrolysis 2013, 103, 362–368. [Google Scholar] [CrossRef]
- Wahyudi, A.; Kurniawan, W.; Hinode, H. Study on Deactivation and Regeneration of Modified Red Mud Catalyst Used in Biodiesel Production. Green Sustain. Chem. 2017, 7, 247–258. [Google Scholar] [CrossRef]
- Rammohan, D.; Kishore, N.; Uppaluri, R.V.S. Reaction kinetics and thermodynamic analysis of non-isothermal co-pyrolysis of Delonix regia and tube waste. Bioresour. Technol. Rep. 2022, 18, 101032. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Luo, F.; Yang, X.; Li, X.; Li, S.; Ye, Y.; Wang, D.; Zheng, Z. Selective production of alkanes and fatty alcohol via hydrodeoxygenation of palmitic acid over red mud-supported nickel catalysts. Fuel 2022, 314, 122780. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Lu, X.; Sun, W.; Xu, Y.; Zhou, J. Catalytic Ozonation for Effective Degradation of Coal Chemical Biochemical Tail Water by Mn/Ce@RM Catalyst. Water 2022, 14, 206. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Singh, R.K. Thermolysis of polanga seed cake to bio-oil using semi batch reactor. Fuel 2012, 97, 450–456. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Baloch, H.A.; Siddiqui, M.T.H.; Nizamuddin, S.; Mubarak, N.M.; Khalid, M.; Srinivasan, M.P.; Griffin, G.J. Solvothermal co-liquefaction of sugarcane bagasse and polyethylene under sub-supercritical conditions: Optimization of process parameters. Process. Saf. Environ. Prot. 2020, 137, 300–311. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Saedi, P.; Momeni, T. Applications of Friedel–Crafts reactions in total synthesis of natural products. RSC Adv. 2018, 8, 40061–40163. [Google Scholar] [CrossRef]
- Blechert, S.; Brodschelm, W.; Hölder, S.; Kammerer, L.; Kutchan, T.M.; Mueller, M.J.; Xia, Z.Q.; Zenk, M.H. The octadecanoic pathway: Signal molecules for the regulation of secondary pathways. Proc. Natl. Acad. Sci. USA 1995, 92, 4099–4105. [Google Scholar] [CrossRef]
- Omar, S.; Alsamaq, S.; Yang, Y.; Wang, J. Production of renewable fuels by blending bio-oil with alcohols and upgrading under supercritical conditions. Front. Chem. Sci. Eng. 2019, 13, 702–717. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Dang, Q.; Wang, J.; Chen, W. Upgrading of Bio-oil over Bifunctional Catalysts in Supercritical Monoalcohols. Energy Fuels 2012, 26, 2990–2995. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Konstas, K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Catalytic upgradation of bio-oil over metal supported activated carbon catalysts in sub-supercritical ethanol. J. Environ. Chem. Eng. 2021, 9, 105059. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Kamizaki, Y.-H.; Terai, N.; Taniya, K.; Tsuruya, S.; Nishiyama, S. One-Step Oxidation of Benzene to Phenol over Cu/Ti/HZSM-5 Catalysts. Catal. Lett. 2010, 134, 324–329. [Google Scholar] [CrossRef]








| Properties | Mesua ferrea L. De-Oiled Cake (Present Study) | Groundnut De-Oiled Cake [6] | Jatropha Curcas De-Oiled Cake [18] | Garlic Husk (Present Study) | Rice Husk [19] | Coffee Husk [17] |
|---|---|---|---|---|---|---|
| Moisture content | 4.08 | 5.6 | 0.44 | 6.8 | 8.43 | 9.06 |
| Volatile content | 82.63 | 83 | 79.2 | 71.5 | 68.25 | 77.09 |
| Ash content | 4.82 | 4.8 | 1.5 | 10.3 | 14.83 | 3.55 |
| Fixed carbon | 8.46 | 6.6 | 18.86 | 11.4 | 16.92 | 19.36 |
| C | 48.63 | 46.36 | 53.39 | 42.1 | 39.48 | 46.41 |
| H | 7.38 | 7.015 | 6.81 | 4.6 | 5.71 | 6.33 |
| N | 3.65 | 6.89 | 0.45 | 0.34 | 0.665 | 2.66 |
| S | - | 0.287 | 0.12 | 0.09 | 0.1 | 0.09 |
| O | 40.34 | 39.438 | 29.27 | 52.87 | 54.12 | 44.51 |
| GHV (MJ kg−1) | 18.86 | 15 | 19.11 | 14.01 | 17.34 | 18.5 |
| Cellulose | 56.91 | - | 16.47 | 41.32 | 41.52 | 47.29 |
| Hemicellulose | 29 | - | 50.31 | 29.34 | 14.04 | 15.57 |
| Lignin | 14.09 | - | 25.91 | 17.17 | 33.67 | 27.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Komandur, J.; Chaudhary, V.; Mohanty, K. Catalytic Co-Pyrolysis of Mesua ferrea L. De-Oiled Cake and Garlic Husk in the Presence of Red-Mud-Based Catalysts. Catalysts 2023, 13, 1401. https://doi.org/10.3390/catal13111401
Kumar A, Komandur J, Chaudhary V, Mohanty K. Catalytic Co-Pyrolysis of Mesua ferrea L. De-Oiled Cake and Garlic Husk in the Presence of Red-Mud-Based Catalysts. Catalysts. 2023; 13(11):1401. https://doi.org/10.3390/catal13111401
Chicago/Turabian StyleKumar, Abhishek, Janaki Komandur, Vasu Chaudhary, and Kaustubha Mohanty. 2023. "Catalytic Co-Pyrolysis of Mesua ferrea L. De-Oiled Cake and Garlic Husk in the Presence of Red-Mud-Based Catalysts" Catalysts 13, no. 11: 1401. https://doi.org/10.3390/catal13111401