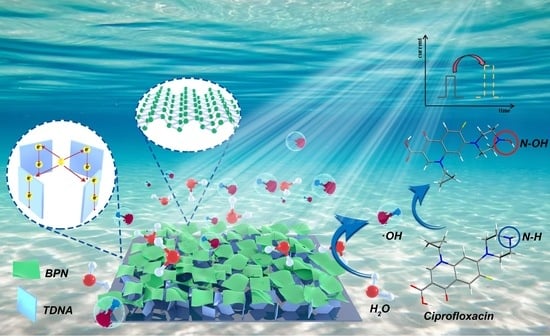
Detection of Ciprofloxacin Based on BPN/TDNA Photoelectrode
Abstract
:1. Introduction
2. Results
2.1. Structure and Morphology of the As-Synthesized Electrodes
2.2. Optimization of Experimental Conditions
2.3. Detection and Analysis of Cip
2.4. Selectivity, Stability, and Reproducibility of BPN/TDNA Electrode for Cip
2.5. Actual Sample Testing
3. Materials and Methods
3.1. Materials
3.2. Synthesis of BPN/TDNA/FTO Composites
3.2.1. Treatment of FTO
3.2.2. Preparation of BPN
3.2.3. Preparation of BPN/TDNA/FTO Photoelectrode
3.3. Apparatus
3.4. Photoelectrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Han, J.; Wang, Y.; Sheng, C.; Liu, Y.; Zhang, G.; Yan, Y. Separation, enrichment and determination of ciprofloxacin using thermoseparating polymer aqueous two-phase system combined with high performance liquid chromatography in milk, egg, and shrimp samples. Food Chem. 2014, 148, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.R.; Peggins, J.O. Simple, rapid determination of enrofloxacin and ciprofloxacin in bovine milk and plasma by high-performance liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2004, 35, 143–153. [Google Scholar] [CrossRef]
- Bojer, C.; Schöbel, J.; Martin, T.; Ertl, M.; Schmalz, H.; Breu, J. Clinical wastewater treatment: Photochemical removal of an anionic antibiotic (ciprofloxacin) by mesostructured high aspect ratio ZnO nanotubes. Appl. Catal. B Environ. 2017, 204, 561–565. [Google Scholar] [CrossRef]
- Martin Santos, A.; Wong, A.; Araujo Almeida, A.; Fatibello-Filho, O. Simultaneous determination of paracetamol and ciprofloxacin in biological fluid samples using a glassy carbon electrode modified with graphene oxide and nickel oxide nanoparticles. Talanta 2017, 174, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Zhang, Z.; Zhu, C.; Tian, Y.; Ye, J. Evolution of microbial communities during electrokinetic treatment of antibiotic-polluted soil. Ecotoxicol. Environ. Saf. 2018, 148, 842–850. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, H.; Zhang, K.; Xu, X.; Wang, C.; Zhu, W.; Wu, W. Long-term low dissolved oxygen accelerates the removal of antibiotics and antibiotic resistance genes in swine wastewater treatment. Chem. Eng. J. 2018, 334, 630–637. [Google Scholar] [CrossRef]
- Alsager, O.A.; Alnajrani, M.N.; Alhazzaa, O. Decomposition of antibiotics by gamma irradiation: Kinetics, antimicrobial activity, and real application in food matrices. Chem. Eng. J. 2018, 338, 548–556. [Google Scholar] [CrossRef]
- Xu, X.; Liu, L.; Jia, Z.; Shu, Y. Determination of enrofloxacin and ciprofloxacin in foods of animal origin by capillary electrophoresis with field amplified sample stacking-sweeping technique. Food Chem. 2015, 176, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gayen, P.; Chaplin, B.P. Selective Electrochemical Detection of Ciprofloxacin with a Porous Nafion/Multiwalled Carbon Nanotube Composite Film Electrode. ACS Appl. Mater. Interfaces 2016, 8, 1615–1626. [Google Scholar] [CrossRef]
- Miao, Y.; Shao, M. Photoelectrocatalysis for high-value-added chemicals production. Chin. J. Catal. 2022, 43, 595–610. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.-C.; Wang, S.; Sillanpää, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Li, D.; Xing, Y.; Zhang, X.; Liu, Y. Bi4TaO8Cl/Bi heterojunction enables high-selectivity photothermal catalytic conversion of CO2-H2O flow to liquid alcohol. Chem. Eng. J. 2022, 435, 135133. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Wang, Q.; Jiang, X.; Shen, Y. Enhanced photoelectrochemical water splitting using a cobalt-sulfide-decorated BiVO4 photoanode. Chin. J. Catal. 2022, 43, 433–441. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, Y.; Yan, K.; Zhang, J. Dual-mode visible light-induced aptasensing platforms for bleomycin detection based on CdS-In(2)S(3) heterojunction. Biosens. Bioelectron. 2019, 145, 111712. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yu, Y.; Guan, Y.; Li, Y.; Wang, B.; Zhu, M. Two-dimensional TiO2 (001) nanosheets as an effective photo-assisted recyclable sensor for the electrochemical detection of bisphenol A. Chin. Chem. Lett. 2020, 31, 2839–2842. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Chen, T.; Hu, L.; Du, Y.; Yao, Y.; Goh, M.C. Simply synthesized nitrogen-doped graphene quantum dot (NGQD)-modified electrode for the ultrasensitive photoelectrochemical detection of dopamine. Nanophotonics 2020, 9, 3831–3839. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zha, Q.; Zhai, C.; Li, W.; Zeng, L.; Zhu, M. Photo-electrochemical detection of dopamine in human urine and calf serum based on MIL-101 (Cr)/carbon black. Microchim. Acta 2020, 187, 526. [Google Scholar] [CrossRef] [PubMed]
- Wageh, S.; Al-Ghamdi, A.A.; Xu, Q. Core-Shell Au@NiS1+x Cocatalyst for Excellent TiO2 Photocatalytic H2 Production. Acta Phys.-Chim. Sin. 2022, 38, 1000–6818. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, C.; Li, D.; Wang, R.; Liang, S.; Li, Y.; Liu, Y.; Zhang, X. Solution Plasma Processing Single-Atom Au1 on CeO2 Nanosheet for Low Temperature Photo-Enhanced Mars–van Krevelen CO Oxidation. Adv. Funct. Mater. 2022, 32, 2207694. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Wang, Z.; Li, J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phosphorene—The two-dimensional black phosphorous: Properties, synthesis and applications (Review). Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, Y.; Dong, F. Advances in Regulation Strategies for Electronic Structure and Performance of Two-Dimensional Photocatalytic Materials. Acta Phys. Chim. Sin. 2020, 37, 2010010. [Google Scholar] [CrossRef]
- Batmunkh, M.; Shrestha, A.; Bat-Erdene, M.; Nine, M.J.; Shearer, C.J.; Gibson, C.T.; Slattery, A.D.; Tawfik, S.A.; Ford, M.J.; Dai, S.; et al. Electrocatalytic Activity of a 2D Phosphorene-Based Heteroelectrocatalyst for Photoelectrochemical Cells. Angew. Chem.-Int. 2018, 57, 2644–2647. [Google Scholar] [CrossRef]
- Tawfik, S.A.; Ali, S.; Fronzi, M.; Kianinia, M.; Tran, T.T.; Stampfl, C.; Aharonovich, I.; Toth, M.; Ford, M.J. First principles investigation of quantum emission from hBN defects. Condens. Matter 2017, 2, 13575–13582. [Google Scholar] [CrossRef] [PubMed]
- Borah, C.K.; Tyagi, P.K.; Kumar, S.; Patel, K. Few-layer p-type phosphorene sheet: An efficient transparent conducting electrode in silicon heterojunction solar cell. Comput. Mater. Sci. 2018, 151, 65–72. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Yu, T.; Li, X. Fabrication of g-C3N4/TiO2 hierarchical spheres with reactive {001} TiO2 crystal facets and its visible-light photocatalytic activity. Int. J. Hydrogen Energy 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Cai, W.; Li, Z.; Liu, J.; Qiu, S.; Pan, Y.; Xu, Z.; Ma, C.; Hu, Y. Recyclable and removable functionalization based on Diels-Alder reaction of black phosphorous nanosheets and its dehydration carbonization in fire safety improvement of polymer composites. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106157. [Google Scholar] [CrossRef]
- Reli, M.; Huo, P.; Šihor, M.; Ambrožová, N.; Troppová, I.; Matějová, L.; Lang, J.; Svoboda, L.; Kuśtrowski, P.; Ritz, M.; et al. Novel TiO2/C3N4 Photocatalysts for Photocatalytic Reduction of CO2 and for Photocatalytic Decomposition of N2O. J. Phys. Chem. A 2016, 120, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.H.; Liu, S.L.; Sim, L.C.; Saravanan, P.; Jang, M.; Ibrahim, S. Surface reconstruction of titania with g-C3N4 and Ag for promoting efficient electrons migration and enhanced visible light photocatalysis. Appl. Surf. Sci. 2015, 358, 370–376. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Jin, M.; Kempa, K.; Shui, L. Water Splitting Performance Enhancement of the TiO2 Nanorod Array Electrode with Ultrathin Black Phosphorus Nanosheets. ChemElectroChem 2020, 7, 96–104. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, T.; Ding, S.; Wei, J.; Yang, G. Anchoring Tailored Low-Index Faceted BiOBr Nanoplates onto TiO2 Nanorods to Enhance the Stability and Visible-Light-Driven Catalytic Activity(Article). ACS Appl. Mater. Interfaces 2017, 9, 16091–16102. [Google Scholar] [CrossRef]
- Koci, K.; Troppova, I.; Reli, M.; Matejova, L.; Edelmannova, M.; Drobna, H.; Dubnova, L.; Rokicinska, A.; Kustrowski, P.; Capek, L. Nd/TiO2 Anatase-Brookite Photocatalysts for Photocatalytic Decomposition of Methanol. Front. Chem. 2018, 6, 2296–2646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tao, L.; Zhao, J.; Yue, X.; Deng, W.; Li, Y.; Wang, C. TiO2/g-C3N4 nanosheets hybrid photocatalyst with enhanced photocatalytic activity under visible light irradiation. Res. Chem. Intermed. 2015, 42, 3609–3624. [Google Scholar] [CrossRef]
- Xu, Z.; Zhuang, C.; Zou, Z.; Wang, J.; Xu, X.; Peng, T. Enhanced photocatalytic activity by the construction of a TiO2/carbon nitride nanosheets heterostructure with high surface area via direct interfacial assembly. Nano Res. 2017, 10, 2193–2209. [Google Scholar] [CrossRef]
- Guo, Y.N.; Xu, G.F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium(III) single molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef]
- Li, G.R.; Wang, F.; Jiang, Q.W.; Gao, X.P.; Shen, P.W. Carbon nanotubes with titanium nitride as a low-cost counter-electrode material for dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2010, 49, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, J.; Guan, J.; Liu, C.; Zhao, X.; Zhu, Z.; Ma, C.; Huo, P.; Li, C.; Yan, Y. Improved photoelectric performance via fabricated heterojunction g-C3N4/TiO2/HNTs loaded photocatalysts for photodegradation of ciprofloxacin. J. Ind. Eng. Chem. 2018, 64, 206–218. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, D.; Yan, Z.; Liu, D.; Qian, K.; Xie, J. A new visible light active multifunctional ternary composite based on TiO2–In2O3 nanocrystals heterojunction decorated porous graphitic carbon nitride for photocatalytic treatment of hazardous pollutant and H2 evolution. Appl. Catal. B Environ. 2015, 170–171, 195–205. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Jun, S.E.; Lee, S.A.; Lee, T.H.; Jang, H.W. Influence of C3N4 Precursors on Photoelectrochemical Behavior of TiO2/C3N4 Photoanode for Solar Water Oxidation. Energies 2020, 13, 974. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ortega, D.; Meléndez, A.M.; Acevedo-Peña, P.; González, I.; Arroyo, R. Semiconducting properties of ZnO/TiO2 composites by electrochemical measurements and their relationship with photocatalytic activity. Electrochim. Acta 2014, 140, 541–549. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Zheng, X. Different inactivation behaviors and mechanisms of representative pathogens (Escherichia coli bacteria, human adenoviruses and Bacillus subtilis spores) in g-C3N4-based metal-free visible-light-enabled photocatalytic disinfection. Sci. Total Env. 2021, 755 Pt 1, 142588. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Chen, S.M. Robust and selective electrochemical detection of antibiotic residues: The case of integrated lutetium vanadate/graphene sheets architectures. J. Hazard. Mater. 2020, 384, 121304. [Google Scholar] [CrossRef]
- He, Z.-L.; Yuan, C.; Gao, H.; Mou, Z.; Qian, S.; Zhai, C.; Lu, C. Significantly Enhanced Photoelectrocatalytic Alcohol Oxidation Performance of CdS Nanowire-Supported Pt via the “Bridge” Role of Nitrogen-Doped Graphene Quantum Dots. ACS Sustain. Chem. Eng. 2020, 8, 12331–12341. [Google Scholar] [CrossRef]
- Yan, P.; Xu, L.; Jiang, D.; Li, H.; Xia, J.; Zhang, Q.; Hua, M.; Li, H. Photoelectrochemical monitoring of ciprofloxacin based on metallic Bi self-doping BiOBr nanocomposites. Electrochim. Acta 2018, 259, 873–881. [Google Scholar] [CrossRef]
- Shan, J.; Li, R.; Yan, K.; Zhu, Y.; Zhang, J. In situ anodic stripping of Cd(II) from CdS quantum dots for electrochemical sensing of ciprofloxacin. Sens. Actuators B Chem. 2016, 237, 75–80. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, F.; Wang, H.; Gao, L.; Wang, Z. A Sensor Based on Au Nanoparticles/Carbon Nitride/Graphene Composites for the Detection of Chloramphenicol and Ciprofloxacin. ECS J. Solid State Sci. Technol. 2018, 7, M201–M208. [Google Scholar] [CrossRef]
- You, F.; Wen, Z.; Yuan, R.; Ding, L.; Wei, J.; Qian, J.; Long, L.; Wang, K. Selective and ultrasensitive detection of ciprofloxacin in milk using a photoelectrochemical aptasensor based on Ti3C2/Bi4VO8Br/TiO2 nanocomposite. J. Electroanal. Chem. 2022, 914, 116285. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Zaeimbashi, R.; Sheikhshoaei, M.; Askari, M.B.; Salarizadeh, P. An electrochemical sensor based on V2O5 nanoparticles for the detection of ciprofloxacin. J. Mater. Sci. Mater. Electron. 2021, 32, 17558–17567. [Google Scholar] [CrossRef]
- Fotouhi, L.; Alahyari, M. Electrochemical behavior and analytical application of ciprofloxacin using a multi-walled nanotube composite film-glassy carbon electrode. Colloids Surf. B Biointerfaces 2010, 81, 110–114. [Google Scholar] [CrossRef]
- Ražná, K.; Hlavačková, L.; Bežo, M.; Žiarovská, J.; Habán, M.; Sluková, Z.; Pernišová, M. Application of the RAPD and miRNA markers in the genotyping of Silybum marianum (L.) Gaertn. Acta Fytotech. Et Zootech. 2015, 18, 83–89. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Allafchian, A.R.; Mohammadzadeh, R. Characterization of MgFe2O4 Nanoparticles as a Novel Electrochemical Sensor: Application for the Voltammetric Determination of Ciprofloxacin. Anal. Sci. 2012, 28, 705–710. [Google Scholar] [CrossRef]
- DeWitte, B.; Dewulf, J.; Demeestere, K.; Vyvere, V.V.D.; Wispelaere, P.D.; Langenhove, H.V. Ozonation of Ciprofloxacin in Water: HRMS Identification of Reaction Products and Pathways. Environ. Sci. Technol. 2008, 42, 4889–4895. [Google Scholar] [CrossRef]
- Vílchez, J.L.; Araujo, L.; Prieto, A.; Navalón, A. Determination of ciprofloxacin and enoxacin in human serum samples by micellar liquid chromatography. Anal. Chim. Acta 2004, 516, 135–140. [Google Scholar] [CrossRef]
- Yuan, Z.; Duan, J.; Fan, S.; Kong, K. Comparison of an ELISA and a HPLC for Determination of Ciprofloxacin Residues in Pork. Food Agric. Immunol. 2010, 13, 199–204. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, R.C.; Aguilar-Caballos, M.P.; Gómez-Hens, A. Simultaneous determination of ciprofloxacin and tetracycline in biological fluids based on dual-lanthanide sensitised luminescence using dry reagent chemical technology. Anal. Chim. Acta 2003, 494, 55–62. [Google Scholar] [CrossRef]
- Hu, X.; Wei, P.; Catanante, G.; Li, Z.; Marty, J.L.; Zhu, Z. Ultrasensitive ciprofloxacin assay based on the use of a fluorescently labeled aptamer and a nanocomposite prepared from carbon nanotubes and MoSe2. Microchim. Acta 2019, 186, 507. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, Y.; Zhu, X.; Hao, Y.; Ding, Y.; Wei, W.; Wang, Q.; Qu, P.; Xu, M. Smart lanthanide coordination polymer fluorescence probe for mercury(II) determination. Anal. Chim. Acta 2016, 912, 139–145. [Google Scholar] [CrossRef] [PubMed]




















| Electrode | Methods | Linear Range (ng mL−1) | Detection Limit (ng mL−1) | Reference |
|---|---|---|---|---|
| BiPO4/BiOI | Photocurrent | 80–7440 | 8.3 | Zhao et al. (2019) [49] |
| CdS | Electrochemical | 33.14–3313.5 | 7.28 | Shan et al. (2016) [50] |
| g-C3N4/Ti3C2 | Amperometry | 0.133–331.34 | 0.043 | Yuan et al. (2020) [51] |
| Ti3C2/Bi4VO8Br/BPN | Photocurrent | 0.331–497.01 | 0.994 | You et al. (2022) [52] |
| V2O5/SPE | DPV | 13.254–120,939.1 | 3.313 | Tajik et al. (2021) [53] |
| MWCNT/GCE | Electrochemical | 13,253.6–331,340 | 19,880.4 | Fotouhi et al. (2010) [54] |
| DNA-based electrochemical biosensor | Electrochemical | 33.134–33,134 | 33.134 | Ražná et al. (2015) [55] |
| MgFe2O4-MWCNTs | Electrochemical | 33.134–331,340 | 3.313 | ENSAFI et al. (2012) [56] |
| BPN/TDNA | Photocurrent | 1.14–438.86 | 1.14 | This work |
| Methods | Linear Range (ng mL−1) | Detection Limit (ng mL−1) | Reference |
|---|---|---|---|
| High resolution mass spectrometry | 20–12,500 | 20 | Bavo et al. [57] |
| Micellar liquid chromatographic | 100–5000 | 24 | José et al. [58] |
| HPLC | 10–5120 | 10 | Yuan et al. [59] |
| ELISA | 0.32–5000 | 0.32 | Yuan et al. [59] |
| Luminescence | 30–2500 | 11 | Rodríguez-Díaz et al. [60] |
| Fluorescently labeled aptasensor | 0.63–800 | 0.63 | Hu et al. [61] |
| Fluorescence | 333–133,000 | 260 | Liu et al. [62] |
| Photocurrent | 1.14–438.86 | 1.14 | This work |
| Disruptors | Observed Current (µA) | Interference Effect (%) | SD (n = 3) | RSD (%) (n = 3) | |||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Average | ||||
| Blank | 0.397 | 0.398 | 0.395 | 0.367 | 0 | 0.002 | 0.504 |
| CIP | 0.591 | 0.594 | 0.597 | 0.594 | 100 | 0.003 | 0.505 |
| BPA | 0.447 | 0.449 | 0.448 | 0.448 | 35.683 | 0.001 | 0.223 |
| PBQ | 0.453 | 0.435 | 0.457 | 0.448 | 35.683 | 0.012 | 2.679 |
| RHB | 0.446 | 0.448 | 0.448 | 0.447 | 35.242 | 0.001 | 0.224 |
| BHA | 0.440 | 0.437 | 0.444 | 0.440 | 32.159 | 0.004 | 0.909 |
| SM | 0.473 | 0.468 | 0.463 | 0.468 | 44.493 | 0.005 | 1.068 |
| MXF | 0.499 | 0.484 | 0.476 | 0.486 | 52.423 | 0.012 | 2.469 |
| GEM | 0.516 | 0.494 | 0.485 | 0.498 | 57.709 | 0.016 | 3.213 |
| NOR | 0.550 | 0.495 | 0.486 | 0.510 | 62.996 | 0.035 | 6.863 |
| OFX | 0.492 | 0.480 | 0.472 | 0.481 | 50.220 | 0.010 | 2.079 |
| Samples | Add (ng mL−1) | Found (ng mL−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Milk | 20 | 20.7 | 103.5 | 4.7 |
| 80 | 83.6 | 104.5 | 3.2 | |
| 150 | 144.3 | 96.2 | 4.3 | |
| 200 | 193.5 | 96.75 | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Wei, S.; Ji, Z.; Wang, J. Detection of Ciprofloxacin Based on BPN/TDNA Photoelectrode. Catalysts 2023, 13, 1368. https://doi.org/10.3390/catal13101368
Yuan J, Wei S, Ji Z, Wang J. Detection of Ciprofloxacin Based on BPN/TDNA Photoelectrode. Catalysts. 2023; 13(10):1368. https://doi.org/10.3390/catal13101368
Chicago/Turabian StyleYuan, Jiangnan, Shusheng Wei, Zhiheng Ji, and Juan Wang. 2023. "Detection of Ciprofloxacin Based on BPN/TDNA Photoelectrode" Catalysts 13, no. 10: 1368. https://doi.org/10.3390/catal13101368