A Review of the Single-Step Flame Synthesis of Defective and Heterostructured TiO2 Nanoparticles for Photocatalytic Applications
Abstract
:1. Introduction
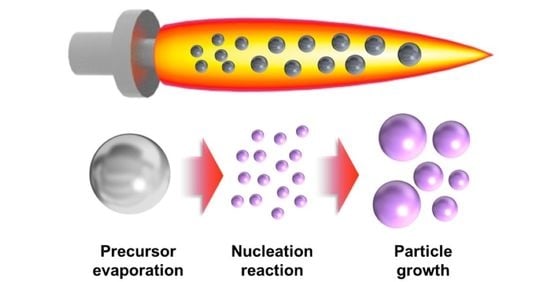
2. Flame Synthesis (FS)
3. Defects and Doping
3.1. Native Defects
3.2. Impurity Doping
| Materials | Precursor/Concentration/Feeding Rate | Gas | Product’s Properties | Photocatalysis | Reference |
|---|---|---|---|---|---|
| Ti3+-TiO2 | TiCl4/0.01~0.2 mol·h−1 | H2/O2/N2 | spherical; sizes (20–40 nm); A-R (A: 68.7%); bandgap (3.07 eV); SSA (42.3 m2·g−1); production rate~3.2 g·h−1 | MB degradation and PEC under visible light (>400 nm); max. photocurrent: 1090 nA·cm−2 | [84] |
| TiO2-x | TiCl4/3 mL·h−1 | C2H4/O2/Ar/N2 | spherical; sizes (10–20 nm); A-R-TiO2-II (R:70%); bandgap (2.8–3 eV); SSA (100–120 m2·g−1) | H2 generation under visible light (>400 nm) with Pt; max. H2 rate: 960 µmol·g−1·h−1 | [86] |
| M- TiO2 (M = V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Ce, Y and Zr) | TTIP (0.5M)/vanadium (V) tri-i-propoxy oxide/chromium (III) 2-ethylhexanoate/iron (III) naphthenate/cobalt 2-ethylhexanoate/manganese (III) naphthenate/molybdenum 2-ethylhexanoate/nickel (III) naphthenate/copper (II) 2-ethylhexanoate/zirconyl 2-ethylhexanoate/3 mL·min−1 | CH4/O2 | spherical/particulate; sizes (51–99 nm); A-R (A: 12–80%); bandgap (2.37–3.21 eV); SSA (60–108 m2·g−1) | acetonitrile conversion under visible light; max. rate constant of Cr-doped TiO2, k: 0.812 m−3·g−1·h−1 | [88,89] |
| N-TiO2 | TTIP-nitric acid-ethanol-DI-urea/3 mL·min−1 | CH4/O2 | spherical/particulate; sizes (50–300 nm); A-R (A: 47–66%); bandgap (2.47–2.95 eV); SSA (17–38 m2·g−1) | phenol degradation under visible light | [79,97] |
| N-TiO2 | TBT-ethanol (0.5M)/NH3-H2O (28 wt.%, 2 mL·min−1)/5 mL·min−1 | H2/Air/N2 | spherical; sizes (10–30 mn); A-R (A: 90.8%); bandgap (2.90 eV); SSA (45.1 m2·g−1); | N/A | [98] |
| S-TiO2 | TTIP-sulfuric acid-ethanol-DI (0.3M)/3 mL·min−1 | CH4/O2 | spherical; sizes (75–311 nm); A-R (A: 59–66%); bandgap 2.78–2.94 eV; SSA (5–13 m2·g−1); | acetaldehyde degradation under visible light | [95] |
| F-TiO2 | TTIP -propionic acid-xylene/ hexafluorobenzene/4 mL·min−1 | CH4/O2 | A-R (A: 86–91%); SSA (113–117 m2·g−1); UV-vis abs < 400 nm | degradation of FA and TAOH under UV-light (>340 nm); max. rate constant k0FA: 1.89 × 10−7 M·s−1, k0TAOH: 14 nM·s−1 | [94] |
| Pt/TO2 | BTB-ethanol/platinum acetylacetonate/3 mL·min−1 | CH4/O2/Ar | particulate; size (~10 nm); A-R (A: 83–89%); SSA (167 m2·g−1); bandgap (3.07–3.19 eV); | H2 production under Xe lamp (300 W), max. H2-rate: 552.39 µmol·h−1 | [99] |
| Pt/TiOx | TTP/platinum acetylacetonate-xylene-acetonitrile/0.4 L·min−1 (N2 flow) | CH4/O2/N2/H2 | particulate; size (20–50 nm); A-R (A: 69%); SSA (74 m2·g−1); bandgap (2.88 eV) | CO2 reduction under Xe lamp; max. AQY: 1.49%, CH4 selectivity: 81% | [85] |
| Pt/N-TiO2 | TBT-ethanol/chloroplatinic acid/5 mL·min−1 | H2/O2/N2/NH3 | particulate; size (10–25 nm); A-R (A: 70.86%); SSA (61.4 m2·g−1); | N/A | [76] |
| Pt/F-TiO2 | TTIP-propionic acid-xylene (0.6 M)/hexafluorobenzene/hexachloroplatinic acid hydrate/4 mL·min−1 | CH4/O2 | A-R (A: 86–91%); SSA (130–142 m2·g−1); UV-vis abs < 400 nm | methanol steam-reforming under UV-light (>350 nm); max. H2 rate: 22 mmol·h−1·g−1 | [100] |
| Pd/TiO2 | TTIP-ethylhexanoic-acetonitrile (0.159 M)/Pd-acetylacetonate/8 mL·min−1 | CH4/O2 | spherical/particulate; size (11–17 nm); A-R-amorphous (A: 74–86%); SSA (85–116); UV-vis abs < 550 nm | NO removal under solar light; max. NO removal: 67% after 5-h reaction | [101,102] |
| Au/TiO2 | TTIP-xelene-pyridine (0.15 M)/1 % dimethyl-gold (III)-acetylacetonate/3.1 mL·min−1 | CH4/O2 | spherical/particulate; size (10–500 nm); A-R (A: 90 wt.%); size (10~500 nm); SSA (106 m2·g−1) | water-splitting reaction under Hg lamp (330–450 nm); max. H2 rate: 52 µmol·h−1·g−1 | [103] |
| AuPd/TiO2 | TTIP-xylene-acetonitrile (0.5 M)/gold chlorite hydrate/palladium acetylacetonate/5 mL·min−1 | CH4/O2 | spherical/particulate; size (10–30 nm); A-R (major A); SSA (99–152 m2·g−1) | N/A | [104] |
| AuPt/TiO2 | TBT-xylene-ethanol (0.05 mol.)/chloroplatinic acid hexahydrate/chloroauric/5 mL·min−1 | H2/O2 | spherical/particulate; size (20–30 nm); A-R (major A); SSA (58–78 m2·g−1) | N/A | [105] |
| Cu/TiO2 | TBT-ethanol/Cu(NO3)2.3H2O/5 mL·min−1 | CH4/O2 | spherical; A-R (A 90–80%); size (~10 nm); bandgap (3.09–3.15 eV); SSA (94–106 m2.g−1) | CO2 reduction under Xe lamp (300–400 nm); max. AQYCH4: 0.087% and AQYCO: 0.057% | [106] |
| CoPt/TiO2 | TBT-ethanol/Co(NO3)2.6H2O/5 mL·min−1 | H2/O2 | spherical/particulate; size (5–25 nm); A-R (A 69.7%); size (5~25 nm); SSA (60.2 m2·g−1) | N/A | [107] |
4. Heterostructure
4.1. TiO2/Noble Metals/Oxides
4.2. TiO2/Non-Noble Metals/Oxides
4.3. TiO2/Others
5. Summary and Outlooks
- (1)
- For achieving the industrial-scale production of flame-synthesized powder with well-defined characteristics, a deep understanding of the evaporation characteristics of the precursor solution, particle nucleation and growth, fluid–particle dynamics, etc., during flame synthesis is required for the design of reactors.
- (2)
- Since flame synthesis involves the instantaneous evolution of powder, the degree of defects and the amount of dopants introduced in each TiO2 nanoparticle can vary slightly. Therefore, more research on homogenizing the particles when prepared at a larger scale should be conducted.
- (3)
- In terms of energy and environment, methods should be developed to clean or recycle gas that arises during the synthesis steps. In addition, efficient flame reactors should be designed in order to achieve low-energy consumption.
- (4)
- The diversification of precursors for obtaining heterostructured TiO2 nanoparticles is necessary. There could still be more elements that can be introduced for the synthesis of heterostructured TiO2 particles.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.M.; Soares, V.F.; Mohallem, N. Synthesis and characterization of TiO2 nanoparticles. Ceram. Int. 2010, 36, 2047–2053. [Google Scholar] [CrossRef]
- Nature. Tiny Tactics Transform Titanium Dioxide. Nature Potfolio; Springer: Cham, Switzerland, 2022. Available online: https://www.nature.com/articles/d42473-022-00068-3 (accessed on 30 December 2022).
- Grand View Research Inc. Titanium Dioxide Market Size, Share & Trends Analysis Report by Grade (Anatase, Rutile), by Production Process (Sulfate, Chloride), by Application (Paints & Coatings, Plastics), by Region, and Segment Forecasts, 2021–2028; Grand View Resesarch Inc.: San Francisco, CA, USA, 2021; Available online: https://www.grandviewresearch.com/industry-analysis/titanium-dioxide-industry (accessed on 30 December 2022).
- Fact.MR. Titanium Dioxide Industry is Projected to Achieve a Global Market Size of US$ 25 bn by 2027, Currently East Asia Accounts for the Largest Share of over 30%. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/03/17/2404873/0/en/Titanium-Dioxide-Industry-is-Projected-to-Achieve-a-Global-Market-Size-of-US-25-Bn-by-2027-Currently-East-Asia-Accounts-for-the-Largest-Share-of-Over-30.html (accessed on 30 December 2022).
- Promnopas, W.; Promnopas, S.; Phonkhokkong, T.; Thongtem, T.; Boonyawan, D.; Yu, L.; Wiranwetchayan, O.; Phuruangrat, A.; Thongtem, S. Crystalline phases and optical properties of titanium dioxide films deposited on glass substrates by microwave method. Surf. Coat. Technol. 2016, 306, 69–74. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Shen, W. Tuning the shape and crystal phase of TiO2 nanoparticles for catalysis. Chem. Commun. 2021, 57, 6838–6850. [Google Scholar] [CrossRef] [PubMed]
- Dette, C.; Pérez-Osorio, M.; Kley, C.; Punke, P.; Patrick, C.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, S.H.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nature 2020, 586, 549–554. [Google Scholar] [CrossRef]
- Chong, R.; Li, J.; Ma, Y.; Zhang, B.; Han, H.; Li, C. Selective conversion of aqueous glucose to value-added sugar aldose on TiO2-based photocatalysts. J. Catal. 2014, 314, 101–108. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Hunge, Y.; Yadav, A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination. J. Colloid. Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2–ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ. Sci.Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.; Mao, S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Choi, H.; Moon, S.-I.; Song, T.; Kim, S. Hydrogen-free defects in hydrogenated black TiO2. Phys. Chem. Chem. Phys. 2018, 20, 19871–19876. [Google Scholar] [CrossRef]
- Khan, S.; Je, M.; Kim, D.; Lee, S.; Cho, S.-H.; Song, T.; Choi, H. Mapping point defects of brookite TiO2 for photocatalytic activity beyond anatase and P25. J. Phys. Chem. C 2020, 124, 10376–10384. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Khan, S.; Choi, H.; Kim, D.; Lee, S.Y.; Zhu, Q.; Zhang, J.; Kim, S.; Cho, S.-H. Self-assembled heterojunction of metal sulfides for improved photocatalysis. Chem. Eng. J. 2020, 395, 125092. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Khan, S.; Choi, J.; Dinh, D.T.; Lee, S.Y.; Paik, U.; Cho, S.-H.; Kim, S. Synergetic control of band gap and structural transformation for optimizing TiO2 photocatalysts. Appl. Catal. B-Environ. 2017, 210, 513–521. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P. Band alignment of rutile and anatase TiO2. Nature Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Konishi, Y.; Tada, H.; Tohge, N.; Nishii, J.; Ito, S. A patterned TiO2 (anatase)/TiO2(rutile) bilayer-type photocatalyst: Effect of the anatase/rutile junction on the photocatalytic activity. Angew. Chem. Int. Ed. 2002, 114, 2935–2937. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.-C.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Liu, H.; Qu, J. Hierarchical nanotubular anatase/rutile/TiO2(B) heterophase junction with oxygen vacancies for enhanced photocatalytic H2 production. Langmuir 2018, 34, 1883–1889. [Google Scholar] [CrossRef]
- Jimmy, C.Y.; Yu, J.; Ho, W.; Zhang, L. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem. Commun. 2001, 1942–1943. [Google Scholar] [CrossRef]
- Marschall, R. Heterojunctions in composite photocatalysts. In Solar Energy for Fuels; Springer: Cham, Switzerland, 2015; pp. 143–172. [Google Scholar] [CrossRef]
- Romero-Morán, A.; Sánchez-Salas, J.L.; Molina-Reyes, J. Influence of selected reactive oxygen species on the photocatalytic activity of TiO2/SiO2 composite coatings processed at low temperature. Appl. Catal. B-Environ. 2021, 291, 119685. [Google Scholar] [CrossRef]
- Rani, S.; Garg, A.; Singh, N. Photocatalytic degradation and mineralization of amoxicillin and ofloxacin using TiO2-SiO2 composites. Toxicol. Environ. Chem. 2021, 103, 137–153. [Google Scholar] [CrossRef]
- Robles-Melgarejo, M.; Espino-Valencia, J.; Natividad-Rangel, R.; Guevara-Martínez, S.J.; Rico-Cerda, J.L.; Rangel-Segura, R. Monoliths of TiO2-SiO2: Synthesis, characterization and photocatalytic activity. J. Porous Mater. 2021, 28, 1697–1711. [Google Scholar] [CrossRef]
- Han, S.; Yu, L.; Zhang, H.; Chu, Z.; Chen, X.; Xi, H.; Long, J. Gold plasmon-enhanced solar hydrogen production over SrTiO3/TiO2 heterostructures. Chem. Cat. Chem. 2019, 11, 6203–6207. [Google Scholar] [CrossRef]
- Wysmulek, K.; Sar, J.; Osewski, P.; Orlinski, K.; Kolodziejak, K.; Trenczek-Zajac, A.; Radecka, M.; Pawlak, D.A. A SrTiO3-TiO2 eutectic composite as a stable photoanode material for photoelectrochemical hydrogen production. Appl. Catal. B-Environ. 2017, 206, 538–546. [Google Scholar] [CrossRef]
- Boro, B.; Gogoi, B.; Rajbongshi, B.; Ramchiary, A. Nano-structured TiO2/ZnO nanocomposite for dye-sensitized solar cells application: A review. Renew. Sustain. Energy Rev. 2018, 81, 2264–2270. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of the TiO2–ZnO heterostructure on the photoinduced hydrophilic conversion of TiO2 and ZnO Surfaces. J. Phys. Chem. C 2019, 123, 8884–8891. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n–p–n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef]
- Yaacob, N.; Sean, G.P.; Nazri, N.A.M.; Ismail, A.F.; Abidin, M.N.Z.; Subramaniam, M.N. Simultaneous oily wastewater adsorption and photodegradation by ZrO2–TiO2 heterojunction photocatalysts. J. Water Process Eng. 2021, 39, 101644. [Google Scholar] [CrossRef]
- Fang, C.; Jiang, X.; Hu, J.; Song, J.; Sun, N.; Zhang, D.; Kuai, L. Ru nanoworms loaded TiO2 for their catalytic performances toward CO oxidation. ACS Appl. Mater. Interfaces 2021, 13, 5079–5087. [Google Scholar] [CrossRef]
- Moon, Y.K.; Jeong, S.Y.; Jo, Y.M.; Jo, Y.K.; Kang, Y.C.; Lee, J.H. Highly selective detection of benzene and discrimination of volatile aromatic compounds using oxide chemiresistors with tunable Rh-TiO2 catalytic overlayers. Adv. Sci. 2021, 8, 2004078. [Google Scholar] [CrossRef]
- Rozman, N.; Nadrah, P.; Cornut, R.; Jousselme, B.; Bele, M.; Dražić, G.; Gaberšček, M.; Kunej, Š.; Škapin, A.S. TiO2 photocatalyst with single and dual noble metal co-catalysts for efficient water splitting and organic compound removal. Int. J. Hydrogen Energy 2021, 46, 32871–32881. [Google Scholar] [CrossRef]
- Xia, C.; Nguyen, T.H.C.; Nguyen, X.C.; Kim, S.Y.; Nguyen, D.L.T.; Raizada, P.; Singh, P.; Nguyen, V.-H.; Nguyen, C.C.; Van Le, Q. Emerging cocatalysts in TiO2-based photocatalysts for light-driven catalytic hydrogen evolution: Progress and perspectives. Fuel 2022, 307, 121745. [Google Scholar] [CrossRef]
- Khan, S.; Kubota, Y.; Lei, W.; Suzuki, N.; Nakata, K.; Terashima, C.; Matsushita, N.; Fujishima, A.; Katsumata, K.-I. One-pot synthesis of (anatase/bronze-type)-TiO2/carbon dot polymorphic structures and their photocatalytic activity for H2 generation. Appl. Surf. Sci. 2020, 526, 146650. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; You, T.; Shi, W.; Li, J.; Guo, L. Au/TiO2/Au as a plasmonic coupling photocatalyst. J. Phys. Chem. C 2012, 116, 6490–6494. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.; Krishnan, V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small 2021, 18, 2101638. [Google Scholar] [CrossRef]
- Khan, S.; Park, B.-I.; Han, J.S.; Lee, S.Y.; Cho, S.-H. Flame synthesized Y2O3:Tb3+–Yb3+ phosphors as spectral convertors for solar cells. Res. Chem. Intermed. 2018, 44, 4619–4632. [Google Scholar] [CrossRef]
- Khan, S.; Han, J.S.; Lee, S.Y.; Cho, S.-H. Flame-synthesized Y2O3:Tb3+ nanocrystals as spectral converting materials. Nanopart. Res. 2018, 20, 241. [Google Scholar] [CrossRef]
- Khan, S.; Katsumata, K.-I.; Rodríguez-González, V.; Terashima, C.; Fujishima, A. Gas-phase synthesis for mass production of TiO2 nanoparticles for environmental applications. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–21. [Google Scholar] [CrossRef]
- Khan, S.; Choi, Y.; Ahn, H.-Y.; Han, J.H.; Ju, B.-K.; Chung, J.; Cho, S.-H. Control of Particle Size in Flame Spray Pyrolysis of Tb–doped Y2O3 for Bio-Imaging. Materials 2020, 13, 2987. [Google Scholar] [CrossRef]
- Cho, S.-H.; Choi, Y.; Lee, S.; Byun; Young, J.; Khan, S. Fabricating Method of Titania Nanoparticles. Korean patent KR102044380B1, 13 November 2019. Available online: https://patents.google.com/patent/KR102044380B1/en?oq=KR102044380B1 (accessed on 30 December 2022).
- Park, J.-S.; Kang, Y.C. Multicomponent (Mo, Ni) metal sulfide and selenide microspheres with empty nanovoids as anode materials for Na-ion batteries. J. Mater. Chem. A 2017, 5, 8616–8623. [Google Scholar] [CrossRef]
- Park, J.-S.; Kang, Y.C. Uniquely structured Sb nanoparticle-embedded carbon/reduced graphene oxide composite shell with empty voids for high performance sodium-ion storage. Chem. Eng. J. 2019, 373, 227–237. [Google Scholar] [CrossRef]
- Park, J.-S.; Yang, S.; Kang, Y.C. Prussian blue analogue nanocubes with hollow interior and porous walls encapsulated within reduced graphene oxide nanosheets and their sodium-ion storage performances. Chem. Eng. J. 2020, 393, 124606. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, J.K.; Hong, J.H.; Cho, J.S.; Park, S.-K.; Kang, Y.C. Advances in the synthesis and design of nanostructured materials by aerosol spray processes for efficient energy storage. Nanoscale 2019, 11, 19012–19057. [Google Scholar] [CrossRef]
- Park, J.-S.; Cho, J.S.; Kim, J.H.; Choi, Y.J.; Kang, Y.C. Electrochemical properties of micron-sized Co3O4 hollow powders consisting of size controlled hollow nanospheres. J. Alloys Compd. 2016, 689, 554–563. [Google Scholar] [CrossRef]
- Sheng, Y.; Kraft, M.; Xu, R. Emerging applications of nanocatalysts synthesized by flame aerosol processes. Curr. Opin. Chem. Eng. 2018, 20, 39–49. [Google Scholar] [CrossRef]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ren, Y.; Biswas, P.; Stephen, D.T. Flame aerosol synthesis of nanostructured materials and functional devices: Processing, modeling, and diagnostics. Prog. Energy Combust. Sci. 2016, 55, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Teoh, W.Y. A perspective on the flame spray synthesis of photocatalyst nanoparticles. Materials 2013, 6, 3194–3212. [Google Scholar] [CrossRef]
- Solakidou, M.; Georgiou, Y.; Deligiannakis, Y. Double-nozzle flame spray pyrolysis as a potent technology to engineer noble metal-TiO2 nanophotocatalysts for efficient H2 production. Energies 2021, 14, 817. [Google Scholar] [CrossRef]
- Huo, J.; Hu, Y.; Jiang, H.; Huang, W.; Li, Y.; Shao, W.; Li, C. Mixed solvents assisted flame spray pyrolysis synthesis of TiO2 hierarchically porous hollow spheres for dye-sensitized solar cells. Ind. Eng. Chem. Res. 2013, 52, 11029–11035. [Google Scholar] [CrossRef]
- Mikaeili, F.; Topcu, S.; Jodhani, G.; Gouma, P.-I. Flame-sprayed pure and Ce-doped TiO2 photocatalysts. Catalysts 2018, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Memarzadeh, S.; Tolmachoff, E.D.; Phares, D.J.; Wang, H. Properties of nanocrystalline TiO2 synthesized in premixed flames stabilized on a rotating surface. Proc. Combust. Inst. 2011, 33, 1917–1924. [Google Scholar] [CrossRef]
- De Falco, G.; Ciardiello, R.; Commodo, M.; Del Gaudio, P.; Minutolo, P.; Porta, A.; D’Anna, A. TiO2 nanoparticle coatings with advanced antibacterial and hydrophilic properties prepared by flame aerosol synthesis and thermophoretic deposition. Surf. Coat. Technol. 2018, 349, 830–837. [Google Scholar] [CrossRef]
- Liu, C.; Camacho, J.; Wang, H. Phase equilibrium of TiO2 nanocrystals in flame-assisted chemical vapor deposition. Chem. Phys. Chem. 2018, 19, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.D.; Park, K.Y.; Jang, H.D. Comparison of titania particles between oxidation of titanium tetrachloride and thermal decomposition of titanium tetraisopropoxide. Aerosol Sci. Technol. 2003, 37, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Liu, M.; Chen, X.; Xu, Z.; Zhao, H. Simultaneous control over lattice doping and nanocluster modification of a hybrid CuOx/TiO2 photocatalyst during flame synthesis for enhancing hydrogen evolution. Solar RRL 2018, 2, 1870234. [Google Scholar] [CrossRef]
- Park, H.K.; Park, K.Y. Control of particle morphology and size in vapor-phase synthesis of titania, silica and alumina nanoparticles. KONA Powder Part. J. 2015, 32, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wang, W.; Tu, W.; Yin, S.; Sheng, Y.; Manuputty, M.Y.; Kraft, M.; Xu, R. Premixed stagnation flame synthesized TiO2 nanoparticles with mixed phases for efficient photocatalytic hydrogen generation. ACS Sustain. Chem. Eng. 2018, 6, 14470–14479. [Google Scholar] [CrossRef]
- Manuputty, M.Y.; Dreyer, J.A.H.; Sheng, Y.; Bringley, E.J.; Botero, M.L.; Akroyd, J.; Kraft, M. Polymorphism of nanocrystalline TiO2 prepared in a stagnation flame: Formation of the TiO2-II phase. Chem. Sci. 2019, 10, 1342–1350. [Google Scholar] [CrossRef] [Green Version]
- Bi, W.; Hu, Y.; Jiang, H.; Yu, H.; Li, W.; Li, C. In-situ synthesized surface N-doped Pt/TiO2 via flame spray pyrolysis with enhanced thermal stability for CO catalytic oxidation. Appl. Surf. Sci. 2019, 481, 360–368. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Yang, F.; Zhao, H. Flame spray pyrolysis synthesized CuO-TiO2 nanoparticles for catalytic combustion of lean CO. Proc. Combust. Instit. 2019, 37, 5499–5506. [Google Scholar] [CrossRef]
- Akurati, K.K.; Vital, A.; Dellemann, J.-P.; Michalow, K.; Graule, T.; Ferri, D.; Baiker, A. Flame-made WO3/TiO2 nanoparticles: Relation between surface acidity, structure and photocatalytic activity. Appl. Catal. B-Environ. 2008, 79, 53–62. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Boningari, T.; Inturi, S.N.R. Single-step synthesis of N-doped TiO2 by flame aerosol method and the effect of synthesis parameters. Aerosol Sci. Technol. 2018, 52, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Smirniotis, P.G.; Boningari, T.; Damma, D.; Inturi, S.N.R. Single-step rapid aerosol synthesis of N-doped TiO2 for enhanced visible light photocatalytic activity. Catal. Commun. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Irfan, A.; Azam, M. Extraction of cobalt ions from aqueous solution by microgels for in-situ fabrication of cobalt nanoparticles to degrade toxic dyes: A two fold-environmental application. Chem. Phys. Lett. 2020, 754, 137645. [Google Scholar] [CrossRef]
- Arif, M.; Farooqi, Z.H.; Irfan, A.; Begum, R. Gold nanoparticles and polymer microgels: Last five years of their happy and successful marriage. J. Mol. Liq. 2021, 336, 116270. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Hu, Y.; Jiang, H.; Li, C. In situ surface hydrogenation synthesis of Ti3+ self-doped TiO2 with enhanced visible light photoactivity. Nanoscale 2014, 6, 9078–9084. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Kavadiya, S.; He, X.; Wang, W.-N.; Karakocak, B.B.; Lin, Y.-C.; Berezin, M.Y.; Biswas, P. Engineering stable Pt nanoparticles and oxygen vacancies on defective TiO2 via introducing strong electronic metal-support interaction for efficient CO2 photoreduction. Chem. Eng. J. 2019, 389, 123450. [Google Scholar] [CrossRef]
- Wu, S.; Manuputty, M.Y.; Sheng, Y.; Wang, H.; Yan, Y.; Kraft, M.; Xu, R. Flame synthesized blue TiO2−x with tunable oxygen vacancies from surface to grain boundary to bulk. Small Methods 2021, 5, 2000928. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Stabilization of Cr in Ti/Si/Cr ternary composites by aerosol flame spray-assisted synthesis for visible-light-driven photocatalysis. Ind. Eng. Chem. Res. 2016, 55, 11839–11849. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B-Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Flame aerosol synthesized Cr incorporated TiO2 for visible light photodegradation of gas phase acetonitrile. J. Phys. Chem. C 2014, 118, 231–242. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Sahle-Demessie, E.; Aly Hassan, A. Selective oxidation using flame aerosol synthesized iron and vanadium-doped nano-TiO2. J. Nanotechnol. 2011, 2011, 209150. [Google Scholar] [CrossRef] [Green Version]
- Teleki, A.; Bjelobrk, N.; Pratsinis, S.E. Flame-made Nb-and Cu-doped TiO2 sensors for CO and ethanol. Sens. Actuators B Chem. 2008, 130, 449–457. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L.; Pratsinis, S.E. Flame sprayed visible light-active Fe-TiO2 for photomineralisation of oxalic acid. Catal. Today 2007, 120, 203–213. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, H.; Li, Y.; Wang, B.; Zhang, L.; Li, C.; Wang, Y.; Cohen, T.; Jiang, Y.; Biswas, P. Engineering the outermost layers of TiO2 nanoparticles using in situ Mg doping in a flame aerosol reactor. AIChE J. 2017, 63, 870–880. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Zuliani, A.; Grigioni, I.; Chiarello, G.L.; Meda, L.; Selli, E. Photocatalytic activity of one step flame-made fluorine doped TiO2. Appl. Catal. A-General 2016, 521, 220–226. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel one-step synthesis of sulfur doped-TiO2 by flame spray pyrolysis for visible light photocatalytic degradation of acetaldehyde. Chem. Eng. J. 2018, 339, 249–258. [Google Scholar] [CrossRef]
- Pennington, A.M.; Halim, H.; Shi, J.; Kear, B.H.; Celik, F.E.; Tse, S.D. Low-pressure flame synthesis of carbon-stabilized TiO2-II (srilankite) nanoparticles. J. Aerosol Sci. 2021, 156, 105775. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel one-step synthesis of nitrogen-doped TiO2 by flame aerosol technique for visible-light photocatalysis: Effect of synthesis parameters and secondary nitrogen (N) source. Chem. Eng. J. 2018, 350, 324–334. [Google Scholar] [CrossRef]
- Huo, J.; Hu, Y.; Jiang, H.; Hou, X.; Li, C. Continuous flame synthesis of near surface nitrogen doped TiO2 for dye-sensitized solar cells. Chem. Eng. J. 2014, 258, 163–170. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Zhao, H. Flame spray pyrolysis made Pt/TiO2 photocatalysts with ultralow platinum loading and high hydrogen production activity. Proc. Combust. Instit. 2021, 38, 6503–6511. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Scavini, M.; Grunwaldt, J.-D.; Selli, E. One step flame-made fluorinated Pt/TiO2 photocatalysts for hydrogen production. Appl. Catal. B-Environ. 2014, 160–161, 144–151. [Google Scholar] [CrossRef]
- Fujiwara, K.; Müller, U.; Pratsinis, S.E. Pd subnano-clusters on TiO2 for solar-light removal of NO. ACS Catal. 2016, 6, 1887–1893. [Google Scholar] [CrossRef]
- Fujiwara, K.; Pratsinis, S.E. Single Pd atoms on TiO2 dominate photocatalytic NOx removal. Appl. Catal. B-Environ. 2018, 226, 127–134. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic hydrogen production over flame spray pyrolysis-synthesised TiO2 and Au/TiO2. Appl. Catal. B-Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Pongthawornsakun, B.; Mekasuwandumrong, O.; Santos Aires, F.J.C.; Büchel, R.; Baiker, A.; Pratsinis, S.E.; Panpranot, J. Variability of particle configurations achievable by 2-nozzle flame syntheses of the Au-Pd-TiO2 system and their catalytic behaviors in the selective hydrogenation of acetylene. Appl. Catal. A-General 2018, 549, 1–7. [Google Scholar] [CrossRef]
- Jiang, J.; Lei, J.; Hu, Y.; Bi, W.; Xu, N.; Li, Y.; Chen, X.; Jiang, H.; Li, C. Electron transfer effect from Au to Pt in Au-Pt/TiO2 towards efficient catalytic activity in CO oxidation at low temperature. Appl. Surf. Sci. 2020, 521, 146447. [Google Scholar] [CrossRef]
- Xiong, Z.; Xu, Z.; Li, Y.; Dong, L.; Wang, J.; Zhao, J.; Chen, X.; Zhao, Y.; Zhao, H.; Zhang, J. Incorporating highly dispersed and stable Cu+ into TiO2 lattice for enhanced photocatalytic CO2 reduction with water. Appl. Surf. Sci. 2020, 507, 145095. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, Y.; Jiang, H.; Yu, J.; Jiang, R.; Li, C. Engineering TiO2 supported Pt sub-nanoclusters via introducing variable valence Co ion in high-temperature flame for CO oxidation. Nanoscale 2018, 10, 13384–13392. [Google Scholar] [CrossRef]
- Davydov, L.; Reddy, E.P.; France, P.; Smirniotis, P.G. Transition-metal-substituted titania-loaded MCM-41 as photocatalysts for the degradation of aqueous organics in visible light. J. Catal. 2001, 203, 157–167. [Google Scholar] [CrossRef]
- Reddy, E.P.; Sun, B.; Smirniotis, P.G. Transition metal modified TiO2-loaded MCM-41 catalysts for visible- and UV-light driven photodegradation of aqueous organic pollutants. J. Phys. Chem. B 2004, 108, 17198–17205. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Effect of the Cr6+ concentration in Cr-incorporated TiO2-loaded MCM-41 catalysts for visible light photocatalysis. Appl. Catal. B-Environ. 2005, 57, 139–149. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Fundamentals and misconceptions in photocatalysis. J. Photochem. Photobiol. A-Chem. 2010, 216, 85–93. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. TiO2-loaded Cr-modified molecular sieves for 4-chlorophenol photodegradation under visible light. J. Catal. 2006, 237, 314–321. [Google Scholar] [CrossRef]
- Chaisuk, C.; Wehatoranawee, A.; Preampiyawat, S.; Netiphat, S.; Shotipruk, A.; Mekasuwandumrong, O. Preparation and characterization of CeO2/TiO2 nanoparticles by flame spray pyrolysis. Ceram. Int. 2011, 37, 1459–1463. [Google Scholar] [CrossRef]
- Michalow-Mauke, K.A.; Lu, Y.; Kowalski, K.; Graule, T.; Nachtegaal, M.; Kröcher, O.; Ferri, D. Flame-made WO3/CeOx-TiO2 catalysts for selective catalytic reduction of NOx by NH3. ACS Catal. 2015, 5, 5657–5672. [Google Scholar] [CrossRef]
- Bahmanrokh, G.; Cazorla, C.; Mofarah, S.S.; Shahmiri, R.; Yao, Y.; Ismail, I.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Band gap engineering of Ce-doped anatase TiO2 through solid solubility mechanisms and new defect equilibria formalism. Nanoscale 2020, 12, 4916–4934. [Google Scholar] [CrossRef]
- Yoo, M.; Yu, Y.-S.; Ha, H.; Lee, S.; Choi, J.-S.; Oh, S.; Kang, E.; Choi, H.; An, H.; Lee, K.-S. A tailored oxide interface creates dense Pt single-atom catalysts with high catalytic activity. Energy Environ. Sci. 2020, 13, 1231–1239. [Google Scholar] [CrossRef]
- Choi, H.; Shin, D.; Yeo, B.C.; Song, T.; Han, S.S.; Park, N.; Kim, S. Simultaneously controllable doping sites and the activity of a W–N codoped TiO2 Photocatalyst. ACS Catal. 2016, 6, 2745–2753. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel continuous single-step synthesis of nitrogen-modified TiO2 by flame spray pyrolysis for photocatalytic degradation of phenol in visible light. J. Mater. Sci. Technol. 2018, 34, 1494–1502. [Google Scholar] [CrossRef]
- Pisduangdaw, S.; Mekasuwandumrong, O.; Fujita, S.-I.; Arai, M.; Yoshida, H.; Panpranot, J. One step synthesis of Pt–Co/TiO2 catalysts by flame spray pyrolysis for the hydrogenation of 3-nitrostyrene. Catal. Commun. 2015, 61, 11–15. [Google Scholar] [CrossRef]
- Ernst, F.O.; Büchel, R.; Strobel, R.; Pratsinis, S.E. One-step flame-synthesis of carbon-embedded and-supported platinum clusters. Chem. Mater. 2008, 20, 2117–2123. [Google Scholar] [CrossRef]
- Fujiwara, K.; Pratsinis, S.E. Atomically dispersed Pd on nanostructured TiO2 for NO removal by solar light. AIChE J. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Mekasuwandumrong, O.; Phothakwanpracha, S.; Jongsomjit, B.; Shotipruk, A.; Panpranot, J. Liquid-phase selective hydrogenation of 1-heptyne over Pd/TiO2 catalyst synthesized by one-step flame spray pyrolysis. Catal. Lett. 2010, 136, 164–170. [Google Scholar] [CrossRef]
- Zong, Y.; Li, S.; Niu, F.; Yao, Q. Direct synthesis of supported palladium catalysts for methane combustion by stagnation swirl flame. Proc. Combust. Instit. 2015, 35, 2249–2257. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Simultaneous deposition of Au nanoparticles during flame synthesis of TiO2 and SiO2. J. Mater. Res. 2003, 18, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Pisduangdaw, S.; Mekasuwandumrong, O.; Yoshida, H.; Fujita, S.-I.; Arai, M.; Panpranot, J. Flame-made Pt/TiO2 catalysts for the liquid-phase selective hydrogenation of 3-nitrostyrene. Appl. Catal. A-General 2015, 490, 193–200. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Mädler, L.; Amal, R. Inter-relationship between Pt oxidation states on TiO2 and the photocatalytic mineralisation of organic matters. J. Catal. 2007, 251, 271–280. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Ninsonthi, H.; Liewhiran, C.; Wisitsoraat, A.; Sriwichai, S.; Phanichphant, S. Flame-made Pt-loaded TiO2 thin films and their application as H2 gas sensors. J. Nanomater. 2013, 2013, 497318. [Google Scholar] [CrossRef] [Green Version]
- Teoh, W.Y.; Mädler, L.; Beydoun, D.; Pratsinis, S.E.; Amal, R. Direct (one-step) synthesis of TiO2 and Pt/TiO2 nanoparticles for photocatalytic mineralisation of sucrose. Chem. Eng. Sci. 2005, 60, 5852–5861. [Google Scholar] [CrossRef]
- Johannessen, T.; Koutsopoulos, S. One-step flame synthesis of an active Pt/TiO2 Catalyst for SO2 oxidation—A possible alternative to traditional methods for parallel screening. J. Catal. 2002, 205, 404–408. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Sriwichai, S.; Phanichphant, S. H2 sensor based on Au/TiO2 nanoparticles by flame spray pyrolysis. Eng. J. 2012, 16, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Himabindu, V.; Srilatha, K.; Bhagawan, D.; Srinivasulu, D. Comparison study between Ni/TiO2 and Ni/flame synthesized TiO2 catalysts for hydrogen production using thermocatalytic decomposition of methane. S. Afr. J. Chem. Eng. 2018, 25, 91–97. Available online: https://hdl.handle.net/10520/EJC-1034aff3ba (accessed on 30 December 2022).
- Tian, X.; Su, M.; Zhao, H. Kinetics of redox reactions of CuO@TiO2–Al2O3 for chemical looping combustion and chemical looping with oxygen uncoupling. Combust. Flame 2020, 213, 255–267. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, H. Low-temperature complete removal of toluene over highly active nanoparticles CuO-TiO2 synthesized via flame spray pyrolysis. Appl. Catal. B-Environ 2020, 264, 118427. [Google Scholar] [CrossRef]
- Akurati, K.K.; Vital, A.; Hany, R.; Bommer, B.; Graule, T.; Winterer, M. One-step flame synthesis of SnO2/TiO2 composite nanoparticles for photocatalytic applications. Int. J. Photoenergy 2005, 7, 153–161. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E.; Baiker, A. Oxidative dehydrogenation of ethane with CO2 over flame-made Ga-loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Shrestha, K.M.; Sorensen, C.M.; Klabunde, K.J. MgO–TiO2 mixed oxide nanoparticles: Comparison of flame synthesis versus aerogel method; characterization, and photocatalytic activities. J. Mater. Res. 2013, 28, 431–439. [Google Scholar] [CrossRef]
- Liu, J.; He, G.; Lu, N.; Li, J. Fabrication of photo-absorption enhanced black TiO2–SiO2 by flame spraying. Mater. Res. Express 2017, 4, 125503. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Y.; Attoui, M.; Chadha, T.S.; Ray, J.R.; Wang, W.-N.; Jun, Y.-S.; Biswas, P. Measurement of sub-2 nm clusters of pristine and composite metal oxides during nanomaterial synthesis in flame aerosol reactors. Anal. Chem. 2014, 86, 7523–7529. [Google Scholar] [CrossRef] [PubMed]
- Kho, E.T.; Lovell, E.; Wong, R.J.; Scott, J.; Amal, R. Manipulating ceria-titania binary oxide features and their impact as nickel catalyst supports for low temperature steam reforming of methane. Appl. Catal. A-General 2017, 530, 111–124. [Google Scholar] [CrossRef]
- Ramadhan, Z.R.; Yun, C.; Park, B.-I.; Yu, S.; Kang, M.H.; Kim, S.; Lim, D.; Choi, B.H.; Han, J.W.; Kim, Y.H. High performance electrochromic devices based on WO3TiO2 nanoparticles synthesized by flame spray pyrolysis. Opt. Mater. 2019, 89, 559–562. [Google Scholar] [CrossRef]
- Bubenhofer, S.B.; Schumacher, C.M.; Koehler, F.M.; Luechinger, N.A.; Grass, R.N.; Stark, W.J. Large-scale synthesis of PbS–TiO2 heterojunction nanoparticles in a single step for solar cell application. J. Phys. Chem. C 2012, 116, 16264–16270. [Google Scholar] [CrossRef]
- Hou, D.; Mao, Q.; Ren, Y.; Luo, K.H. Atomic insights into mechanisms of carbon coating on titania nanoparticle during flame synthesis. Carbon 2023, 201, 189–199. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Park, J.-S.; Ishihara, T. A Review of the Single-Step Flame Synthesis of Defective and Heterostructured TiO2 Nanoparticles for Photocatalytic Applications. Catalysts 2023, 13, 196. https://doi.org/10.3390/catal13010196
Khan S, Park J-S, Ishihara T. A Review of the Single-Step Flame Synthesis of Defective and Heterostructured TiO2 Nanoparticles for Photocatalytic Applications. Catalysts. 2023; 13(1):196. https://doi.org/10.3390/catal13010196
Chicago/Turabian StyleKhan, Sovann, Jin-Sung Park, and Tatsumi Ishihara. 2023. "A Review of the Single-Step Flame Synthesis of Defective and Heterostructured TiO2 Nanoparticles for Photocatalytic Applications" Catalysts 13, no. 1: 196. https://doi.org/10.3390/catal13010196