Integrated Photocatalytic Oxidation and Adsorption Approach for the Robust Treatment of Refinery Wastewater Using Hybrid TiO2/AC
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of TiO2/AC Hybrid Adsorbent
2.2. Adsorption over AC
2.3. Adsorption Kinetics
2.4. Adsorption Isotherms
2.5. Thermodynamic Parameters
2.6. Simultaneous Oxidation and Adsorption with TiO2/AC
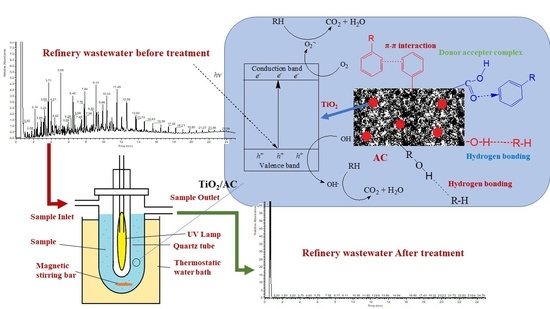
2.7. Treatment of Refinery Wastewater
2.8. GC-MS Analysis
2.9. Process Mechanism
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Preparation and Characterization of TiO2/AC Hybrid Adsorbent
3.3. Characterization of TiO2/AC
3.4. Adsorption Experiments
3.5. Integrated Photocatalytic Oxidation-Adsorption Experiments
3.6. Sample Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suleĭmanov, R.; Gabbasova, I.; Sitdikov, R. Changes in the properties of oily gray forest soil during biological reclamation. Izv. Akad. Nauk. Seriia Biol. 2005, 32, 109–115. [Google Scholar] [CrossRef]
- Wake, H. Oil refineries: A review of their ecological impacts on the aquatic environment. Estuar. Coast. Shelf Sci. 2005, 62, 131–140. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Gao, F.; Zhou, J.; Cen, K. The slurrying properties of slurry fuels made of petroleum coke and petrochemical sludge. Fuel Process. Technol. 2012, 104, 57–66. [Google Scholar] [CrossRef]
- Ye, G.; Lu, X.; Han, P.; Peng, F.; Wang, Y.; Shen, X. Application of ultrasound on crude oil pretreatment. Chem. Eng. Process. Process Intensif. 2008, 47, 2346–2350. [Google Scholar] [CrossRef]
- Stepnowski, P.; Siedlecka, E.; Behrend, P.; Jastorff, B. Enhanced photo-degradation of contaminants in petroleum refinery wastewater. Water Res. 2002, 36, 2167–2172. [Google Scholar] [CrossRef]
- Coelho, A.; Castro, A.V.; Dezotti, M.; Sant’Anna Jr, G. Treatment of petroleum refinery sourwater by advanced oxidation processes. J. Hazard. Mater. 2006, 137, 178–184. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2014, 2, 56–62. [Google Scholar] [CrossRef]
- World Energy Outlook 2019; IEA (International Energy Agency): Paris, France, 2019.
- Saien, J.; Nejati, H. Enhanced photocatalytic degradation of pollutants in petroleum refinery wastewater under mild conditions. J. Hazard. Mater. 2007, 148, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.R.; Tambade, P.S.; Chaskar, M.G.; Gadave, K.M. Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO2: A comparative study. Drink. Water Eng. Sci. 2017, 10, 109–117. [Google Scholar] [CrossRef] [Green Version]
- El-Naas, M.H.; Surkatti, R.; Al-Zuhair, S. Petroleum refinery wastewater treatment: A pilot scale study. J. Water Process. Eng. 2016, 14, 71–76. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Zhang, L.; Chen, M.; Ahmad, F.; Fida, H.; Zhang, H. Heterogeneous Activation of Peroxymonosulfate by a Spinel CoAl2O4 Catalyst for the Degradation of Organic Pollutants. Catalysts 2022, 12, 847. [Google Scholar] [CrossRef]
- Lv, N.; Wang, X.; Peng, S.; Zhang, H.; Luo, L. Study of the kinetics and equilibrium of the adsorption of oils onto hydrophobic jute fiber modified via the sol-gel method. Int. J. Environ. Res. Public Health. 2018, 15, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channa, A.M.; Baytak, S.; Memon, S.Q.; Talpur, M.Y. Equilibrium, kinetic and thermodynamic studies of removal of phenol from aqueous solution using surface engineered chemistry. Heliyon 2019, 5, e01852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.V.; de Castro, M.M.; Martinez-Escandell, M.; Molina-Sabio, M.; Rodriguez-Reinoso, F. A continuous binding site affinity distribution function from the Freundlich isotherm for the supercritical adsorption of hydrogen on activated carbon. J. Phys. Chem. C 2010, 114, 13759–13765. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- Rathee, G.; Awasthi, A.; Sood, D.; Tomar, R.; Tomar, V.; Chandra, R. A new biocompatible ternary Layered Double Hydroxide Adsorbent for ultrafast removal of anionic organic dyes. Sci. Rep. 2019, 9, 16225. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, M.; Mazaheri, H.; Khodadoust, S.; Hajati, S.; Purkait, M. Application of central composite design for simultaneous removal of methylene blue and Pb2+ ions by walnut wood activated carbon. Spectrochim. Acta—A Mol. Biomol. 2015, 135, 479–490. [Google Scholar] [CrossRef]
- Delaune, R.; Lindau, C.; Jugsujinda, A. Effectiveness of “Nochar” solidifier polymer in removing oil from open water in coastal wetlands. Spill Sci. Technol. Bull. 1999, 5, 357–359. [Google Scholar] [CrossRef]
- Pelletier, É.; Siron, R. Silicone-based polymers as oil spill treatment agents. Environ. Toxicol. Chem. 1999, 18, 813–818. [Google Scholar] [CrossRef]
- Lim, T.-T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic–oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Nguyen, H.-T.T.; Thi Cam Nguyen, D.; TN Le, H.; Thi Nguyen, T.; Thi Hong Le, N.; Lim, K.T.; Duy Nguyen, T.; Tran, T.V.; Bach, L.G. Process Optimization by a Response Surface Methodology for Adsorption of Congo Red Dye onto Exfoliated Graphite-Decorated MnFe2O4 Nanocomposite: The Pivotal Role of Surface Chemistry. Processes 2019, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Lupandina, N.; Sapronova, Z.A. Modified Bleaching Clay as a Sorption Material. E&ES 2020, 459, 042063. [Google Scholar]
- Pereira, K.R.d.O.; Hanna, R.A.; Vianna, M.M.G.R.; Pinto, C.A.; Rodrigues, M.G.F.; Valenzuela-Diaz, F.R. Brazilian organoclays as nanostructured sorbents of petroleum-derived hydrocarbons. Mater. Res. 2005, 8, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Giusti, D.; Conway, R.; Lawson, C. Activated carbon adsorption of petrochemicals. J. Water Pollut. Control. Fed. 1974, 46, 947–965. [Google Scholar]
- Abussaud, B.; Asmaly, H.A.; Saleh, T.A.; Gupta, V.K.; Atieh, M.A. Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J. Mol. Liq. 2016, 213, 351–359. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Fu, D.; Meng, X.; An, Q.; Chu, P.K. Removal of organic pollutants from super heavy oil wastewater by lignite activated coke. Colloids. Surf. A Physicochem. Eng. Asp. 2014, 447, 120–130. [Google Scholar] [CrossRef]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Alhakimi, G.; Studnicki, L.H.; Al-Ghazali, M. Photocatalytic destruction of potassium hydrogen phthalate using TiO2 and sunlight: Application for the treatment of industrial wastewater. J. Photochem. Photobiol. A Chem. 2003, 154, 219–228. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.; Zhang, H.; Yang, W.; Li, J.; Zhou, K. Enhanced photocatalytic degradation of organic contaminants over CaFe2O4 under visible LED light irradiation mediated by peroxymonosulfate. J. Mater. Sci. Technol. 2021, 62, 34–43. [Google Scholar] [CrossRef]
- Haarstrick, A.; Kut, O.M.; Heinzle, E. TiO2-assisted degradation of environmentally relevant organic compounds in wastewater using a novel fluidized bed photoreactor. Environ. Sci. Technol. 1996, 30, 817–824. [Google Scholar] [CrossRef]
- Bahnemann, D.; Kholuiskaya, S.; Dillert, R.; Kulak, A.; Kokorin, A. Photodestruction of dichloroacetic acid catalyzed by nano-sized TiO2 particles. Appl. Catal. B 2002, 36, 161–169. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Zhang, L.; Zhang, J.; Jin, L. Application of nano TiO2 towards polluted water treatment combined with electro-photochemical method. Water Res. 2003, 37, 3815–3820. [Google Scholar] [CrossRef] [PubMed]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Amin, N.; El-Ashtoukhy, E.Z. Electrochemical removal of phenol from oil refinery wastewater. J. Hazard. Mater. 2009, 163, 711–716. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Santos, F.; Azevedo, E.; Dezotti, M. Photocatalysis as a tertiary treatment for petroleum refinery wastewaters. Braz. J. Chem. Eng. 2006, 23, 451–460. [Google Scholar] [CrossRef]
- Li, J.; Song, X.; Hu, G.; Thring, R.W. Ultrasonic desorption of petroleum hydrocarbons from crude oil contaminated soils. J. Environ. Sci. Health A 2013, 48, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Ul haq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M. Photocatalytic oxidative degradation of hydrocarbon pollutants in refinery wastewater using TiO2 as catalyst. Water Environ. Res. 2020, 92, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Đukić, A.B.; Kumrić, K.R.; Vukelić, N.S.; Stojanović, Z.S.; Stojmenović, M.D.; Milošević, S.S.; Matović, L.L. Influence of ageing of milled clay and its composite with TiO2 on the heavy metal adsorption characteristics. Ceram. Int. 2015, 41, 5129–5137. [Google Scholar] [CrossRef]
- Zhu, Z.-l.; Li, A.-m.; Xia, M.-f.; Wan, J.-n.; Zhang, Q.-x. Preparation and characterization of polymer-based spherical activated carbons. Chin. J. Polym. Sci. 2008, 26, 645–651. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Huang, D.; Miyamoto, Y.; Matsumoto, T.; Tojo, T.; Fan, T.; Ding, J.; Guo, Q.; Zhang, D. Preparation and characterization of high-surface-area TiO2/activated carbon by low-temperature impregnation. Sep. Purif. Technol. 2011, 78, 9–15. [Google Scholar] [CrossRef]
- Olafadehan, O.; Aribike, D. Treatment of industrial wastewater effluent. J. Niger. Soc. Chem. Eng. 2000, 19, 50–53. [Google Scholar]
- Daud, W.M.A.W.; Houshamnd, A.H. Textural characteristics, surface chemistry and oxidation of activated carbon. J. Nat. Gas Chem. 2010, 19, 267–279. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Wan Alwi, S.R.; Webb, C.; Ghasemi, N.; Muhamad, I.I. Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel biowaste. J. Clean. Prod. 2016, 118, 210–222. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.; Nasri, N.S.; Zaini, M.A.A.; Hamza, U.D.; Ani, F.N. Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int. Biodeterior. Biodegrad. 2015, 102, 245–255. [Google Scholar] [CrossRef]
- Kilic, M.; Apaydin-Varol, E.; Pütün, A.E. Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2011, 189, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard. Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Dong, Y.; Wang, H.; Liu, Y. Adsorption behavior of ammonium by a bioadsorbent–Boston ivy leaf powder. Res. J. Environ. Sci. 2010, 22, 1513–1518. [Google Scholar] [CrossRef]
- Desta, M.B. Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J. Thermodyn. 2013, 2013, 375830. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-J.; Kang, C.-S.; You, Y.-J.; Chung, M.-C.; Woo, M.-W.; Jeong, W.-J.; Park, N.-C.; Ahn, H.-G. Adsorption–desorption characteristics of VOCs over impregnated activated carbons. Catal. Today 2006, 111, 223–228. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; da Silva, M.L.C.P.; Alvarez-Mendes, M.O.; dos Reis Coutinho, A.; Thim, G.P. Phenol removal from aqueous solution by activated carbon produced from avocado kernel seeds. Chem. Eng. J. 2011, 174, 49–57. [Google Scholar] [CrossRef]
- Tham, Y.; Latif, P.A.; Abdullah, A.; Shamala-Devi, A.; Taufiq-Yap, Y. Performances of toluene removal by activated carbon derived from durian shell. Bioresour. Technol. 2011, 102, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Alkaram, U.F.; Mukhlis, A.A.; Al-Dujaili, A.H. The removal of phenol from aqueous solutions by adsorption using surfactant-modified bentonite and kaolinite. J. Hazard. Mater. 2009, 169, 324–332. [Google Scholar] [CrossRef]
- Brandão, P.C.; Souza, T.C.; Ferreira, C.A.; Hori, C.E.; Romanielo, L.L. Removal of petroleum hydrocarbons from aqueous solution using sugarcane bagasse as adsorbent. J. Hazard. Mater. 2010, 175, 1106–1112. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- El-Gawad, S.; El-Aziz, H. Effective removal of chemical oxygen demand and phosphates from aqueous medium using entrapped activated carbon in alginate. MOJ Biol. Med. 2018, 3, 227–236. [Google Scholar] [CrossRef]
- Sun, W.-l.; Qu, Y.-z.; Yu, Q.; Ni, J.-r. Adsorption of organic pollutants from coking and papermaking wastewaters by bottom ash. J. Hazard. Mater. 2008, 154, 595–601. [Google Scholar] [CrossRef]
- Saien, J.; Shahrezaei, F. Organic pollutants removal from petroleum refinery wastewater with nanotitania photocatalyst and UV light emission. Int. J. Photoenergy 2012, 2012, 703074. [Google Scholar] [CrossRef]
- Pakravan, P.; Akhbari, A.; Moradi, H.; Azandaryani, A.H.; Mansouri, A.M.; Safari, M. Process modeling and evaluation of petroleum refinery wastewater treatment through response surface methodology and artificial neural network in a photocatalytic reactor using poly ethyleneimine (PEI)/titania (TiO2) multilayer film on quartz tube. Appl. Petrochem. Res. 2015, 5, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Bickley, R.; Slater, M.; Wang, W.-J. Engineering development of a photocatalytic reactor for waste water treatment. Process. Saf. Environ. Prot. 2005, 83, 205–216. [Google Scholar] [CrossRef]
- Masschelein, W.; Rice, R. Ultraviolet Light in Water and Wastewater Sanitation; Lewis Publishers: Boca Raton, FL, USA, 2002; pp. 3–4. [Google Scholar]
- Saien, J.; Soleymani, A. Degradation and mineralization of Direct Blue 71 in a circulating upflow reactor by UV/TiO2 process and employing a new method in kinetic study. J. Hazard. Mater. 2007, 144, 506–512. [Google Scholar] [CrossRef]
- Benyahia, F.; Abdulkarim, M.; Embaby, A.; Rao, M. Refinery wastewater treatment: A true technological challenge. In Proceedings of the Seventh Annual UAE University Research Conference, Al-Ain, United Arab Emirates, 22–24 April 2006. [Google Scholar]
- da Silva, L.J.; Alves, F.C.; de França, F.P. A review of the technological solutions for the treatment of oily sludges from petroleum refineries. Waste Manag. Res. 2012, 30, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C.; Balsamo, M.; Erto, A. Mechanisms of Methylparaben Adsorption onto Activated Carbons: Removal Tests Supported by a Calorimetric Study of the Adsorbent-Adsorbate Interactions. Molecules 2019, 24, 413. [Google Scholar] [CrossRef] [Green Version]
- Hami, M.L.; Al-Hashimi, M.; Al-Doori, M. Effect of activated carbon on BOD and COD removal in a dissolved air flotation unit treating refinery wastewater. Desalination 2007, 216, 116–122. [Google Scholar] [CrossRef]
- Ma, F.; Guo, J.B.; Zhao, L.J.; Chang, C.C.; Cui, D. Application of bioaugmentation to improve the activated sludge system into the contact oxidation system treating petrochemical wastewater. Bioresour. Technol. 2009, 100, 597–602. [Google Scholar] [CrossRef] [PubMed]











| pH | Level (mg/L) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| BOD | COD | SS | O&G | Phenols | NH3 | -SO4−2 | ||
| 7–9 | 150–360 | 300–600 | ≤150 | ≤50 | - | 15 | - | [14] |
| 8.0 | 40.25 | 80–120 | 22.8 | - | 13 | - | - | [15] |
| 6.6 | - | 596 | 120 | - | - | - | 887 | [16] |
| 150–350 | 300–800 | 100 | 3000 | 20–300 | - | - | [17] | |
| 8.0 | 570 | 850–1020 | - | 12.7 | 98–128 | 5.1–2.11 | 15–23 | [18] |
| 10 | 8.0 | 80.8 | - | 47.5 | - | 2.3 | - | [17] |
| - | 658–710.5 | - | 45 | 30 | 22 | 10 | [19] | |
| Elements | Mass % | Elements | Mass % |
|---|---|---|---|
| C | 66.65 | Al | 0.38 |
| O | 28.71 | Si | 0.72 |
| Ti | 2.0 | S | 0.12 |
| Ca | 0.90 | Zn | 0.53 |
| Benzene | Toluene | Phenol | Naphthalene | |
|---|---|---|---|---|
| Pseudo-first-order kinetic parameters | ||||
| k1 (mg g−1min−1) | 0.021 | 0.021 | 0.021 | 0.021 |
| qe1 (mg g−1) | 2.761 | 2.576 | 2.600 | 2.612 |
| R2 | 0.930 | 0.931 | 0.930 | 0.933 |
| Pseudo-second-order kinetic parameters | ||||
| k2 (mg g−1min−1) | 0.044 | 0.024 | 0.025 | 0.017 |
| qe2 (mg g−1) | 4.878 | 3.676 | 3.760 | 0.067 |
| R2 | 0.150 | 0.101 | 0.179 | 0.261 |
| Parameters | Benzene | Toluene | Phenol | Naphthalene |
|---|---|---|---|---|
| Langmuir isotherm parameters | ||||
| qm (mg/g) | 4.902 | 3.676 | 3.690 | 4.329 |
| K1 | 0.040 | 0.031 | 0.031 | 0.035 |
| R2 | 0.998 | 0.997 | 0.998 | 0.998 |
| Freundlich isotherm parameters | ||||
| N | 0.988 | 0.752 | 0.753 | 0.873 |
| Kf | 1.834 | 2.018 | 2.017 | 1.915 |
| R2 | 0.997 | 0.998 | 0.999 | 0.999 |
| Temperature (°C) | ΔG° (kJ·mol–1) | ΔH° (kJ·mol–1) | ΔS° (kJ·mol–1) | ΔG° (kJ·mol–1) | ΔH° (kJ·mol–1) | ΔS° (kJ·mol–1) |
|---|---|---|---|---|---|---|
| Benzene | Toluene | |||||
| 30 | 4.560 | −0.0010 | 0.002 | 5.076 | −0.0004 | 0.001 |
| 35 | 3.607 | 4.532 | ||||
| 40 | 3.771 | 4.816 | ||||
| 45 | 3.937 | 5.000 | ||||
| 50 | 4.107 | 5.079 | ||||
| Phenol | Naphthalene | |||||
| 30 | 4.971 | −0.0017 | 0.001 | 4.556 | −0.0007 | 0.001 |
| 35 | 4.635 | 4.224 | ||||
| 40 | 4.922 | 4.710 | ||||
| 45 | 5.101 | 4.893 | ||||
| 50 | 5.299 | 4.970 | ||||
| Sources of Variation | Df | Sum of Squares | Mean Square | F-Value | Prob. Value |
|---|---|---|---|---|---|
| Rep. | 2 | 0.54136 | 0.27068 | ||
| Pollutants | 3 | 140.275 | 46.7583 | 826.99 ** | 0.000 |
| Error I | 6 | 0.33924 | 0.05654 | ||
| Doses | 10 | 104558 | 10455.8 | 307,591 ** | 0.000 |
| Pollutant x Dose | 30 | 149.635 | 4.98751 | 146.72 ** | 0.000 |
| Error-II | 80 | 2.71939 | 0.03399 | ||
| Total | 131 | 104851 |
| Dose | Benzene | Toluene | Phenol | Naphthalene | Mean |
|---|---|---|---|---|---|
| 100 | 10.500i | 10.433i | 11.100h | 10.667i | 10.675K |
| 200 | 23.433f | 21.700g | 23.299f | 21.500g | 22.458J |
| 300 | 33.167b | 30.700d | 32.333c | 29.6333e | 31.458I |
| 400 | 42.700X | 40.167Z | 42.233Y | 39.267a | 41.092H |
| 500 | 51.300T | 49.333V | 50.400U | 48.300W | 49.833G |
| 600 | 59.200R | 58.333S | 60.367Q | 58.267S | 59.042F |
| 700 | 70.233N | 69.333O | 69.300O | 67.567P | 69.108E |
| 800 | 88.567J | 81.233K | 80.600L | 79.300M | 82.425D |
| 900 | 92.133D | 90.200H | 89.733I | 90.600G | 90.667C |
| 1000 | 94.400A | 92.700C | 91.033F | 92.100D | 92.233B |
| 1100 | 94.00B | 92.033DF | 91.133F | 91.767E | 92.558A |
| Mean across all doses | 59.967A | 57.833C | 58.312B | 57.179D |
| Treatment of Refinery Wastewater | Reaction Time (min) | COD (mg/L) | Reduction in COD (%) |
|---|---|---|---|
| Untreated sample | - | 970 | - |
| Adsorption over AC | 105 | 77 | 92 |
| Photocatalytic oxidation by UV/TiO2 | 90 | 65 | 93 |
| Integrated photocatalytic oxidation and adsorption over TiO2/AC | 50 | 48.5 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ul Haq, I.; Ahmad, W.; Ahmad, I.; Shah, A.; Yaseen, M.; Muhammad, T. Integrated Photocatalytic Oxidation and Adsorption Approach for the Robust Treatment of Refinery Wastewater Using Hybrid TiO2/AC. Catalysts 2023, 13, 193. https://doi.org/10.3390/catal13010193
Ul Haq I, Ahmad W, Ahmad I, Shah A, Yaseen M, Muhammad T. Integrated Photocatalytic Oxidation and Adsorption Approach for the Robust Treatment of Refinery Wastewater Using Hybrid TiO2/AC. Catalysts. 2023; 13(1):193. https://doi.org/10.3390/catal13010193
Chicago/Turabian StyleUl Haq, Ihtisham, Waqas Ahmad, Imtiaz Ahmad, Amjad Shah, Muhammad Yaseen, and Taj Muhammad. 2023. "Integrated Photocatalytic Oxidation and Adsorption Approach for the Robust Treatment of Refinery Wastewater Using Hybrid TiO2/AC" Catalysts 13, no. 1: 193. https://doi.org/10.3390/catal13010193