NOx Photooxidation over Different Noble Metals Modified TiO2
Abstract
:1. Introduction
2. Results and Discussion
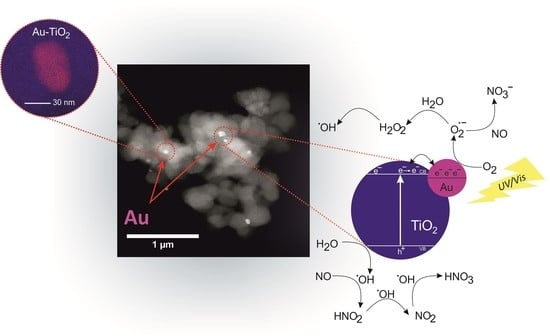
2.1. Catalyst Characterization
2.2. Photocatalytic Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photocatalysts
3.3. Analytical Methods
3.4. Photoactivity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verbruggen, S.W. TiO2 Photocatalysis for the Degradation of Pollutants in Gas Phase: From Morphological Design to Plasmonic Enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Lasek, J.; Yu, Y.H.; Wu, J.C.S. Removal of NOx by Photocatalytic Processes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, B.S.; Huang, C.W.; Le, T.T.; Nguyen, C.C.; Nhi Le, T.T.; Heo, D.; Ly, Q.V.; Trinh, Q.T.; Shokouhimehr, M.; et al. Photocatalytic NOx Abatement: Recent Advances and Emerging Trends in the Development of Photocatalysts. J. Clean. Prod. 2020, 270, 121912. [Google Scholar] [CrossRef]
- Skalska, K.; Ledakowicz, S.; Louwe, R.; Szymczak, R. Nitrogen Oxides Pre-Ozonation in Flue Gases from Phosphate Rock Digestion. Chem. Eng. J. 2017, 318, 181–188. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, S. State-of-the-Art and Current Challenges for TiO2/UV-LED Photocatalytic Degradation of Emerging Organic Micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A Review on Photocatalysis for Air Treatment: From Catalyst Development to Reactor Design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Vasilache, V.; Popa, C.; Vasilache, T. New Materials for Photocatalytic Purification of Air—A Review. Food Environ. Saf. J. 2011, 10, 2–8. [Google Scholar]
- Bumajdad, A.; Madkour, M. Understanding the Superior Photocatalytic Activity of Noble Metals Modified Titania under UV and Visible Light Irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Bowering, N.; Croston, D.; Harrison, P.G.; Walker, G.S. Silver Modified Degussa P25 for the Photocatalytic Removal of Nitric Oxide. Int. J. Photoenergy 2007, 2007, 090752. [Google Scholar] [CrossRef] [Green Version]
- Sofianou, M.V.; Boukos, N.; Vaimakis, T.; Trapalis, C. Decoration of TiO2 Anatase Nanoplates with Silver Nanoparticles on the {1 0 1} Crystal Facets and Their Photocatalytic Behaviour. Appl. Catal. B Environ. 2014, 158–159, 91–95. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Zanella, R.; Aguilar-Elguezabal, A.; Arizabalo, R.D.; Castillo, S.; Moran-Pineda, M. Decomposition of NO in Gas Phase by Gold Nanoparticles Supported on Titanium Dioxide Synthesized by the Deposition–Precipitation Method. Mater. Sci. Eng. B 2010, 174, 13–17. [Google Scholar] [CrossRef]
- Wu, Z.; Sheng, Z.; Liu, Y.; Wang, H.; Tang, N.; Wang, J. Characterization and Activity of Pd-Modified TiO2 Catalysts for Photocatalytic Oxidation of NO in Gas Phase. J. Hazard. Mater. 2009, 164, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wang, I.K.; Lin, Y.M.; Tseng, Y.H.; Lu, C.M. Visible Light Photocatalytic Degradation of Nitric Oxides on PtOx-Modified TiO2 via Sol–Gel and Impregnation Method. J. Mol. Catal. A Chem. 2010, 316, 163–170. [Google Scholar] [CrossRef]
- Hernández Rodríguez, M.J.; Pulido Melián, E.; García Santiago, D.; González Díaz, O.; Navío, J.A.; Doña Rodríguez, J.M. NO Photooxidation with TiO2 Photocatalysts Modified with Gold and Platinum. Appl. Catal. B Environ. 2017, 205, 148–157. [Google Scholar] [CrossRef]
- Zielińska, A.; Kowalska, E.; Sobczak, J.W.; Łacka, I.; Gazda, M.; Ohtani, B.; Hupka, J.; Zaleska, A. Silver-Doped TiO2 Prepared by Microemulsion Method: Surface Properties, Bio- and Photoactivity. Sep. Purif. Technol. 2010, 72, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: EdenPrairie, MN, USA, 1992. [Google Scholar]
- Wängberg, I.; Etzkorn, T.; Barnes, I.; Platt, U.; Becker, K.H. Absolute Determination of the Temperature Behavior of the NO2 + NO3 +(M) ↔ N2O5 + (M) Equilibrium. J. Phys. Chem. A 1997, 101, 9694–9698. [Google Scholar] [CrossRef]
- Kamboures, M.A.; Raff, J.D.; Miller, Y.; Phillips, L.F.; Finlayson-Pitts, B.J.; Gerber, R.B. Complexes of HNO3 and NO3− with NO2 and N2O4, and Their Potential Role in Atmospheric HONO Formation. Phys. Chem. Chem. Phys. 2008, 10, 6019–6032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoa, N.T.; Kim, S.W.; Yoo, D.H.; Kim, E.J.; Hahn, S.H. Size-Dependent Work Function and Catalytic Performance of Gold Nanoparticles Decorated Graphene Oxide Sheets. Appl. Catal. A Gen. 2014, 469, 159–164. [Google Scholar] [CrossRef]
- Cybula, A.; Priebe, J.B.; Pohl, M.M.; Sobczak, J.W.; Schneider, M.; Zielińska-Jurek, A.; Brückner, A.; Zaleska, A. The Effect of Calcination Temperature on Structure and Photocatalytic Properties of Au/Pd Nanoparticles Supported on TiO2. Appl. Catal. B Environ. 2014, 152–153, 202–211. [Google Scholar] [CrossRef]






| Element | Band | Ag 0.1 | Ag 0.5 | Au 0.1 | Au 0.5 | Pt 0.1 | Pt 0.5 | Pd 0.1 | Pd 0.5nr | Pd 0.5r |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (% at.) | ||||||||||
| Titanium | Ti 2p | 22.61 ± 0.14 | 24.04 ± 0.24 | 23.48 ± 0.23 | 22.17 ± 1.45 | 23.39 ± 0.18 | 23.91 ± 0.28 | 24.65 ± 0.24 | 24.37 ± 0.16 | 24.10 ± 0.13 |
| Oxygen | O 1s | 53.80 ± 0.49 | 55.74 ± 0.17 | 56.87 ± 0.04 | 55.05 ± 1.65 | 57.17 ± 1.39 | 57.43 ± 0.50 | 58.64 ± 0.70 | 57.51 ± 0.47 | 56.50 ± 0.01 |
| Carbon | C 1s | 23.56 ± 0.36 | 19.97 ± 0.08 | 19.62 ± 0.26 | 22.74 ± 3.09 | 19.38 ± 1.56 | 18.49 ± 0.78 | 16.68 ± 0.45 | 17.94 ± 0.33 | 19.24 ± 0.11 |
| Metal band | Ag 3d | Au 4f | Pt 4f | Pd 3d | ||||||
| 0.04 ± 0.00 | 0.25 ± 0.02 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.18 ± 0.01 | 0.03 ± 0.02 | 0.18 ± 0.01 | 0.17 ± 0.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalska, K.; Malankowska, A.; Balcerzak, J.; Gazda, M.; Nowaczyk, G.; Jurga, S.; Zaleska-Medynska, A. NOx Photooxidation over Different Noble Metals Modified TiO2. Catalysts 2022, 12, 857. https://doi.org/10.3390/catal12080857
Skalska K, Malankowska A, Balcerzak J, Gazda M, Nowaczyk G, Jurga S, Zaleska-Medynska A. NOx Photooxidation over Different Noble Metals Modified TiO2. Catalysts. 2022; 12(8):857. https://doi.org/10.3390/catal12080857
Chicago/Turabian StyleSkalska, Kinga, Anna Malankowska, Jacek Balcerzak, Maria Gazda, Grzegorz Nowaczyk, Stefan Jurga, and Adriana Zaleska-Medynska. 2022. "NOx Photooxidation over Different Noble Metals Modified TiO2" Catalysts 12, no. 8: 857. https://doi.org/10.3390/catal12080857