The Organic Bromide Sources Adjusting the Shape and Band Structures of BiOBr Nanosheets for Enhanced Photodegradation Performances of BPA
Abstract
:1. Introduction
2. Results and Discussion
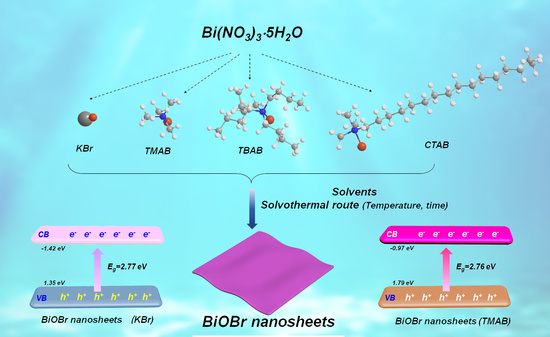
2.1. Effects of Organic Bromide Sources, Solvent, and Concentration on the Shape and Phase Control of BiOBr Nanosheets
2.2. Structure Variations and Photocatalytic Performances of BiOBr Nanosheets
3. Experimental
3.1. Synthesis of BiOBr Nanosheets
3.2. Characterization
3.3. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, H.J.; Huang, H.W.; Xu, K.; Hao, W.C.; Guo, Y.X.; Wang, S.B.; Shen, X.L.; Pan, S.F.; Zhang, Y.H. Liquid-phase exfoliation into monolayered BiOBr nanosheets for photocatalytic oxidation and reduction. ACS Sustain. Chem. Eng. 2017, 5, 10499–10508. [Google Scholar] [CrossRef]
- Ye, L.Q.; Jin, X.L.; Liu, C.; Ding, C.H.; Xie, H.Q.; Chu, K.H.; Wong, P.K. Thickness-ultrathin and bismuth-rich strategies for BiOBr to enhance photoreduction of CO2 into solar fuels. Appl. Catal. B-Environ. 2016, 187, 281–290. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.D.; Shi, W.; Ling, P.Q.; Sun, Y.F.; Jiao, X.C.; Gao, S.; Liang, L.; Xu, J.Q.; Yan, W.S.; et al. Efficient visible-light-driven CO2 reduction mediated by defect-engineered BiOBr atomic layers. Angew. Chem. Int. Edit. 2018, 57, 8719–8723. [Google Scholar] [CrossRef]
- Wu, D.; Ye, L.Q.; Yip, H.Y.; Wong, P.K. Organic-free synthesis of {001} facet dominated BiOBr nanosheets for selective photoreduction of CO2 to CO. Catal. Sci. Technol. 2017, 7, 265–271. [Google Scholar] [CrossRef]
- Feng, H.F.; Xu, Z.F.; Wang, L.; Yu, Y.X.; Mitchell, D.; Cui, D.; Xu, X.; Shi, J.; Sannomiya, T.; Du, Y.; et al. Modulation of photocatalytic properties by strain in 2D BiOBr nanosheets. ACS Appl. Mater. Inter. 2015, 7, 27592–27596. [Google Scholar] [CrossRef]
- Bhachu, D.S.; Moniz, S.J.A.; Sathasivam, S.; Scanlon, D.O.; Walsh, A.; Bawaked, S.M.; Mokhtar, M.; Obaid, A.Y.; Parkin, I.P.; Tang, J.W.; et al. Bismuth oxyhalides: Synthesis, structure and photoelectrochemical activity. Chem. Sci. 2016, 7, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, X.F.; Chen, X.T.; Xue, Z.L. BiOBr hierarchical microspheres: Microwave-assisted solvothermal synthesis, strong adsorption and excellent photocatalytic properties. J. Colloid. Interf. Sci. 2011, 354, 630–636. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Wang, Q.G.; Wu, Q.S. High {001} facets dominated BiOBr lamellas: Facile hydrolysis preparation and selective visible-light photocatalytic activity. J. Mater. Chem. A 2013, 1, 8622–8629. [Google Scholar] [CrossRef]
- Wang, Z.D.; Chu, Z.; Dong, C.W.; Wang, Z.; Yao, S.Y.; Gao, H.; Liu, Z.Y.; Liu, Y.; Yang, B.; Zhang, H. Ultrathin BiOX (X = Cl, Br, I) nanosheets with exposed {001} facets for photocatalysis. ACS Appl. Nano Mater. 2020, 3, 1981–1991. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.Y.; Chen, S.C.; Jiang, S.L.; Zhang, X.D.; Shao, W.; Zhang, Q.; Yan, W.S.; Pan, B.C.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef]
- Kong, X.Y.; Lee, W.P.C.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Oxygen-deficient BiOBr as a highly stable photocatalyst for efficient CO2 reduction into renewable carbon-neutral fuels. ChemCatChem. 2016, 8, 3074–3081. [Google Scholar] [CrossRef]
- Xue, X.L.; Chen, R.P.; Chen, H.W.; Hu, Y.; Ding, Q.Q.; Liu, Z.T.; Ma, L.B.; Zhu, G.Y.; Zhang, W.J.; Yu, Q.; et al. Oxygen vacancy engineering promoted photocatalytic ammonia synthesis on ultrathin two-dimensional bismuth oxybromide nanosheets. Nano Lett. 2018, 18, 7372–7377. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Xiao, X.Y.; Chen, J.Y.; Wang, Y. Fabrication of BiVO4/BiOBr composite with enhanced photocatalytic activity by a CTAB-assisted polyol method. J. Photoch. Photobio. A 2019, 368, 153–161. [Google Scholar] [CrossRef]
- Meng Shang, W.W. Ling Zhang, Preparation of BiOBr lamellar structure with high photocatalytic activity by CTAB as Br source and template. J. Hazard. Mater. 2009, 167, 803–809. [Google Scholar] [CrossRef]
- Xia, J.X.; Ge, Y.P.; Di, J.; Xu, L.; Yin, S.; Chen, Z.G.; Liu, P.J.; Li, H.M. Ionic liquid-assisted strategy for bismuth-rich bismuth oxybromides nanosheets with superior visible light-driven photocatalytic removal of bisphenol-A. J. Colloid. Interf. Sci 2016, 473, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.N.; Zhang, Z.Y.; Yan, J.F.; Zhang, F.C. Effect of In-doping on electronic structure and optical properties of Sr2TiO4. Chin. Phys. Lett. 2009, 26, 067102. [Google Scholar]
- Xing, Y.L.; Wang, J.X.; Chen, L.; Wang, A.Q.; Li, F.; Wang, C.; Zhong, E.Q. Synthesis and characterization of Cu-BiVO4/MCM-41 composite catalysts with enhanced visible light photocatalytic activities. J. Mater. Sci. Mater. El. 2016, 27, 8633–8640. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, M.; Li, Z.; Pan, Y.; Qiu, J.; Li, T.; Xu, K.; Zhang, S.; Xu, D.; Guo, M. Regulating the phase transition of monoclinic Bi4O5Br2 through the synergistic effect of “drag force” and facet recognition by branched polyethyleneimine. Cryst. Eng. Comm. 2020, 22, 5871–5881. [Google Scholar] [CrossRef]
- Wu, Z.H.; Shen, J.; Ma, N.; Li, Z.F.; Wu, M.; Xu, D.F.; Zhang, S.Y.; Feng, W.H.; Zhu, Y.F. Bi4O5Br2 nanosheets with vertical aligned facets for efficient visible-light-driven photodegradation of BPA. Appl. Catal. B-Environ. 2021, 286, 119937. [Google Scholar] [CrossRef]
- Wu, Z.H.; Seok, S.; Kim, D.H.; Kim, W.S. Control of Crystal Size Distribution using Non-Isothermal Taylor Vortex Flow. Cryst. Growth Des. 2015, 15, 5675–5684. [Google Scholar] [CrossRef]
- Liao, C.X.; Ma, Z.J.; Chen, X.F.; He, X.; Qiu, J.R. Controlled synthesis of bismuth oxyiodide toward optimization of photocatalytic performance. Appl. Surf. Sci. 2016, 387, 1247–1256. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, Y.; Zhang, Z.M.; Huang, Z.; Lian, L.Y.; Miao, X.S.; Zhang, D.L.; Beard, M.C.; Zhang, J.B. Combination of cation exchange and quantized Ostwald ripening for controlling size distribution of lead chalcogenide quantum dots. Chem. Mater. 2017, 29, 3615–3622. [Google Scholar] [CrossRef]
- Houk, L.R.; Challa, S.R.; Grayson, B.; Fanson, P.; Datye, A.K. The definition of “critical radius” for a collection of nanoparticles undergoing Ostwald ripening. Langmuir 2009, 25, 11225–11227. [Google Scholar] [CrossRef]
- Dagtepe, P.; Chikan, V. Quantized Ostwald ripening of colloidal nanoparticles. J. Phys. Chem. C. 2010, 114, 16263–16269. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, C.X.; Lu, M.L.; Zhang, L.; Liu, F.; Zuo, X.X.; Nan, J.M. Deficient Bi24O31Br10 as a highly efficient photocatalyst for selective oxidation of benzyl alcohol into benzaldehyde under blue LED irradiation. Appl. Catal. B-Environ. 2018, 228, 142–151. [Google Scholar] [CrossRef]
- Shi, J.L.; Feng, K.; Hao, H.; Ku, C.; Sit, P.H.-L.; Teoh, W.Y.; Lang, X. 2D sp2 Carbon-conjugated covalent organic frameworkwith pyrene-tethered TEMPO intercalation for photocatalytic aerobic oxidation of sulfides into sulfoxides. Sol. RRL 2022, 6, 210060. [Google Scholar]
- Xing, W.; Liu, C.; Zhong, H.; Zhang, Y.; Zhang, T.; Cheng, C.; Han, J.; Wu, G.; Chen, G. Phosphate group-mediated carriers transfer and energy band over carbon nitride for efficient photocatalytic H2 production and removal of rhodamine B. J. Alloy Compd. 2022, 895, 162772. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.Z.; Sun, S.M.; Jiang, D.; Gao, E.P. Selective transport of electron and hole among {001} and {110} facets of BiOCl for pure water splitting. Appl. Catal. B-Environ. 2015, 162, 470–474. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Lv, D.D.; Liu, C.W.; Lai, W.H.; Sun, C.Y.; Su, Z.M.; Xu, X.; Hao, W.C.; Dou, S.X.; et al. Promoted photocharge separation in 2D lateral epitaxial heterostructure for visible-light-driven CO2 photoreduction. Adv. Mater. 2020, 32, 2004311. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.Q.; Zhang, D.; Li, B.; Lv, L.; Wang, Y.; Ren, H.J.; Zhang, X.L.; Xia, A.; Liu, Y. Defect-mediated Z-scheme BiO2-x/Bi2O2.75 photocatalyst for full spectrum solar-driven organic dyes degradation. Appl. Catal. B-Environ. 2019, 254, 98–112. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Li, Y.H.; Qi, M.Y.; Li, J.Y.; Tang, Z.R.; Zhou, Z.; Xu, Y.J. Transition metal doping BiOBr nanosheets with oxygen vacancy and exposed {102} facets for visible light nitrogen fixation. Appl. Catal. B-Environ. 2021, 281, 119516. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Chen, T.; Wang, L.; Shi, X.; Zhang, X.; Chen, D. Facet-dependent photocatalytic N2 fixation of bismuth-rich Bi5O7I nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 27661–27668. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Cao, Y.H.; Zhang, F.Y.; Zou, Y.Z.; Huang, Z.A.; Ye, L.Q.; Zhou, Y. Interfacial oxygen vacancy engineered two-dimensional g-C3N4/BiOCl heterostructures with boosted photocatalytic conversion of CO2. ACS Appl. Energ. Mater. 2020, 3, 4610–4618. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, D.; Sun, K.; Zeng, Y.; Wang, L.; Zhang, Y.; Shen, J.; Wu, Z.; Feng, W. The Organic Bromide Sources Adjusting the Shape and Band Structures of BiOBr Nanosheets for Enhanced Photodegradation Performances of BPA. Catalysts 2022, 12, 820. https://doi.org/10.3390/catal12080820
Xia D, Sun K, Zeng Y, Wang L, Zhang Y, Shen J, Wu Z, Feng W. The Organic Bromide Sources Adjusting the Shape and Band Structures of BiOBr Nanosheets for Enhanced Photodegradation Performances of BPA. Catalysts. 2022; 12(8):820. https://doi.org/10.3390/catal12080820
Chicago/Turabian StyleXia, Donghao, Kaiyang Sun, Youwei Zeng, Lulu Wang, Yi Zhang, Jie Shen, Zhaohui Wu, and Wenhui Feng. 2022. "The Organic Bromide Sources Adjusting the Shape and Band Structures of BiOBr Nanosheets for Enhanced Photodegradation Performances of BPA" Catalysts 12, no. 8: 820. https://doi.org/10.3390/catal12080820