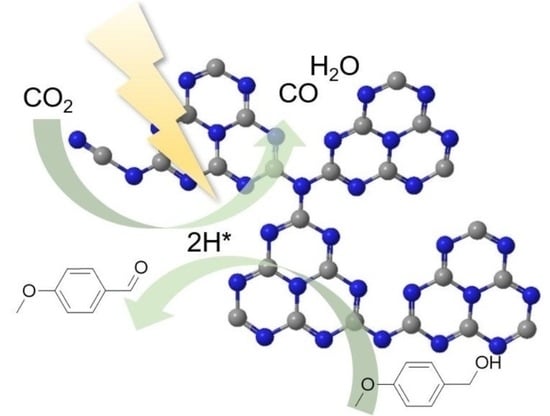
Photocatalytic CO2 Reduction Coupled with Alcohol Oxidation over Porous Carbon Nitride
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Preparation of Carbon Nitride with Porogens
3.2. Photocatalytic Reactions
3.3. Electrochemical and Photoelectrochemical Measurements
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Gu, J.; Hsu, C.S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qiu, C.; Wang, S.; Gao, H.; Yu, K.; Zhang, Z.; Ling, X.; Ou, W.; Su, C. Electrocatalytic water-splitting for the controllable and sustainable synthesis of deuterated chemicals. Sci. Bull. 2021, 66, 562–569. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.B.; Hung, S.F.; Ding, J.; Cai, W.; Liu, L.; Gao, J.; Li, X.; Ren, X.; Kuang, Z.; et al. Elucidating the Electrocatalytic CO2 Reduction Reaction over a Model Single-Atom Nickel Catalyst. Angew. Chem. Int. Ed. 2020, 59, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, T.J.; Feng, W.J.; Liu, Y.X.; Wang, H.H.; Su, H.; Lv, L.B.; Li, X.H.; Chen, J.S. Polarized few-layer g-C3N4 as metal-free electrocatalyst for highly efficient reduction of CO2. Nano Res. 2018, 11, 2450–2459. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, B.; Ran, J.; Davey, K.; Qiao, S.Z. Atomic-Level Reactive Sites for Semiconductor-Based Photocatalytic CO2 Reduction. Adv. Energy Mater. 2020, 10, 1903879. [Google Scholar] [CrossRef]
- Niu, P.; Pan, Z.; Wang, S.; Wang, X. Tuning Crystallinity and Surface Hydrophobicity of a Cobalt Phosphide Cocatalyst to Boost CO2 Photoreduction Performance. ChemSusChem 2021, 14, 1302–1307. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Feng, H.; Guo, Q.; Xu, Y.; Chen, T.; Zhou, Y.; Wang, Y.; Wang, M.; Shen, D. Surface Nonpolarization of g-C3N4 by Decoration with Sensitized Quantum Dots for Improved CO2 Photoreduction. ChemSusChem 2018, 11, 4256–4261. [Google Scholar] [CrossRef]
- Cui, Y.; Ge, P.; Chen, M.; Xu, L. Research Progress in Semiconductor Materials with Application in the Photocatalytic Reduction of CO2. Catalysts 2022, 12, 372. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Liu, T.; Cai, S.; Zou, X.; Jiang, J.; Mei, Z.; Gao, Z.; Guo, H. Rich S vacant g-C3N4@CuIn5S8 hollow heterojunction for highly efficient selective photocatalytic CO2 reduction. Chem. Eng. J. 2021, 424, 130325. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Duan, M.; Xiong, J.; Long, R.; Xu, M.; Kang, L.; Guo, S.; et al. Cobalt nitride as a novel cocatalyst to boost photocatalytic CO2 reduction. Nano Energy 2021, 79, 105429. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, Y.; Liu, D.; Yu, Y.; Zhang, B. Integrating photocatalytic reduction of CO2 with selective oxidation of tetrahydroisoquinoline over InP–In2O3 Z-scheme p-n junction. Sci. China Chem. 2020, 63, 28–34. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.Z.; Chen, S.; Duan, M.; Xiong, J.; Zhu, C.; Long, R.; Hao, W.; Chi, Z.; et al. Isolated single atom cobalt in Bi3O4Br atomic layers to trigger efficient CO2 photoreduction. Nat. Commun. 2019, 10, 2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Karthikeyan, S.; Lee, F. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Heil, T.; Zafeiratos, S.; Lai, F.; Savateev, A.; Antonietti, M.; Wang, X. Tailoring the Grain Boundary Chemistry of Polymeric Carbon Nitride for Enhanced Solar Hydrogen Production and CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 3433–3437. [Google Scholar] [CrossRef]
- Li, A.; Cao, Q.; Zhou, G.; Schmidt, B.V.K.J.; Zhu, W.; Yuan, X.; Huo, H.; Gong, J.; Antonietti, M. Three-Phase Photocatalysis for the Enhanced Selectivity and Activity of CO2 Reduction on a Hydrophobic Surface. Angew. Chem. Int. Ed. 2019, 58, 14549–14555. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Grills, D.C.; Polyansky, D.E.; Szalda, D.J.; Fujita, E. Unexpected Roles of Triethanolamine in the Photochemical Reduction of CO2 to Formate by Ruthenium Complexes. J. Am. Chem. Soc. 2020, 142, 2413–2428. [Google Scholar] [CrossRef]
- Lu, M.; Li, Q.; Liu, J.; Zhang, F.M.; Zhang, L.; Wang, J.L.; Kang, Z.H.; Lan, Y.Q. Installing earth-abundant metal active centers to covalent organic frameworks for efficient heterogeneous photocatalytic CO2 reduction. Appl. Catal. B-Environ. 2019, 254, 624–633. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Yang, P.; Huang, C.; Wang, X. Boron Carbon Nitride Semiconductors Decorated with CdS Nanoparticles for Photocatalytic Reduction of CO2. ACS Catal. 2018, 8, 4928–4936. [Google Scholar] [CrossRef]
- Markushyna, Y.; Lamagni, P.; Catalano, J.; Lock, N.; Zhang, G.; Antonietti, M.; Savateev, A. Advantages in Using Inexpensive CO2 To Favor Photocatalytic Oxidation of Benzylamines. ACS Catal. 2020, 10, 7336–7342. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Qiu, C.; Xu, Y.; Zuo, J. Engineering carbon nitride with cyanide groups for efficient photocatalytic alcohol oxidation and H2O2 production-Utilization of photogenerated electrons and holes. Appl. Surf. Sci. 2022, 573, 151506. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, J.; Chen, S.; Zhang, S.; Huang, W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Meng, S.; Ye, X.; Zhang, J.; Fu, X.; Chen, S. Effective use of photogenerated electrons and holes in a system: Photocatalytic selective oxidation of aromatic alcohols to aldehydes and hydrogen production. J. Catal. 2018, 367, 159–170. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Wang, W. Photon-initiated heterogeneous redox couples for methylation of anilines under mild conditions. Green Chem. 2020, 22, 4433–4437. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, C.; Xu, Y.; Han, Q.; Tang, J.; Loh, K.P.; Su, C. Semiconductor photocatalysis to engineering deuterated N-alkyl pharmaceuticals enabled by synergistic activation of water and alkanols. Nat. Commun. 2020, 11, 4722. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Chen, Q.; Zhao, Y.; Zeng, L.; Yang, C.; Huang, F.; Huang, L. Sulfate modified g-C3N4 with enhanced photocatalytic activity towards hydrogen evolution: The role of sulfate in photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 10116–10122. [Google Scholar] [CrossRef]
- Shaya, J.; Srour, H.; Karamé, I. An outline of carbon dioxide chemistry, uses and technology. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Karamé, I., Shaya, J., Srour, H., Eds.; InTech Open: London, UK, 2018; Online publication; Chapter 1; pp. 4–13. [Google Scholar]
- Nanda, S.; Vo, D.V.; Nguyen, V.H. Carbon Dioxide Capture and Conversion. In Advanced Materials and Processes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 1; pp. 10–18. [Google Scholar]
- Huang, P.; Huang, J.; Li, J.; Pham, T.D.; Zhang, L.; He, J.; Brudvig, G.W.; Deskins, N.A.; Frenkel, A.I.; Li, G. Revealing the structure of single cobalt sites in carbon nitride for photocatalytic CO2 reduction. J. Phys. Chem. C 2022, 126, 8596–8604. [Google Scholar] [CrossRef]
- Guo, H.; Niu, C.G.; Liang, C.; Niu, H.Y.; Yang, Y.Y.; Liu, H.Y.; Tang, N.; Fang, H.X. Highly crystalline porous carbon nitride with electron accumulation capacity: Promoting exciton dissociation and charge carrier generation for photocatalytic molecular oxygen activation. Chem. Eng. J. 2021, 409, 128030. [Google Scholar] [CrossRef]
- Huang, C.; Wen, Y.; Ma, J.; Dong, D.; Shen, Y.; Liu, S.; Ma, H.; Zhang, Y. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, M.; Yang, W.; Xiao, Y.; Zeng, L.; Wu, X.; Xu, Q.; Su, C.; He, Q. Homogeneous carbon/potassium-incorporation strategy for synthesizing red polymeric carbon nitride capable of near-infrared photocatalytic H2 production. Adv. Mater. 2021, 33, 2101455. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Shi, J. Defect engineering of photocatalysts towards elevated CO2 reduction performance. ChemSusChem 2021, 14, 2635–2654. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, L.; Wang, M.; Tian, J.; Jin, X.; Guo, L.; Wang, L.; Shi, J. Carbon-vacancy modified graphitic carbon nitride: Enhanced CO2 photocatalytic reduction performance and mechanism probing. J. Mater. Chem. A 2019, 7, 1556–1563. [Google Scholar] [CrossRef]
- Guo, L.; You, Y.; Huang, H.; Tian, N.; Ma, T.; Zhang, Y. Z-scheme g-C3N4/Bi2O2[BO2(OH)] heterojunction for enhanced photocatalytic CO2 reduction. J. Colloid Interface Sci. 2020, 568, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ma, H.; Zhang, W.; Zhu, G.; Yang, W.; Son, N.; Kang, M.; Liu, C. Z-scheme SnFe2O4-graphitic carbon nitride: Reusable, magnetic catalysts for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2020, 383, 123172. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, X.; Qin, H.; Wang, F.; Li, Y.; Li, X.; Kang, S.; Zuo, Y.; Cui, L. Facile One-Step Synthesis of Hybrid Graphitic Carbon Nitride and Carbon Composites as High-Performance Catalysts for CO2 Photocatalytic Conversion. ACS Appl. Mater. Interfaces 2016, 8, 17212–17219. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-Doped Graphitic Carbon Nitride Nanotubes with Amino-rich Surface for Efficient CO2 Capture, Enhanced Photocatalytic Activity, and Product Selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Yang, Y.; Li, F.; Chen, J.; Fan, J.; Xiang, Q. Single Au Atoms Anchored on Amino-Group-Enriched Graphitic Carbon Nitride for Photocatalytic CO2 Reduction. ChemSusChem 2020, 13, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhu, B.; Cao, S.; Yu, J. Controlling defects in crystalline carbon nitride to optimize photocatalytic CO2 reduction. Chem. Commun. 2020, 56, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, X.; Zhang, X.; Wu, Y.; Ma, C.; Huo, P.; Yan, Y. Fabricating C and O co-doped carbon nitride with intramolecular donor-acceptor systems for efficient photoreduction of CO2 to CO. Appl. Catal. B Environ. 2020, 268, 118736. [Google Scholar] [CrossRef]
- Jiang, K.; Zhu, L.; Wang, Z.; Liu, K.; Li, H.; Hu, J.; Pan, H.; Fu, J.; Zhang, N.; Qiu, X.; et al. Plasma-treatment induced H2O dissociation for the enhancement of photocatalytic CO2 reduction to CH4 over graphitic carbon nitride. Appl. Surf. Sci. 2020, 508, 145173. [Google Scholar] [CrossRef]
- Li, F.; Yue, X.; Zhang, D.; Fan, J.; Xiang, Q. Targeted regulation of exciton dissociation in graphitic carbon nitride by vacancy modification for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 292, 120179. [Google Scholar] [CrossRef]
- Wang, Y.; Zhen, W.; Zeng, Y.; Wan, S.; Guo, H.; Zhang, S.; Zhong, Q. In situ self-assembly of zirconium metal–organic frameworks onto ultrathin carbon nitride for enhanced visible light-driven conversion of CO2 to CO. J. Mater. Chem. A 2020, 8, 6034–6040. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Wang, S.; Zuo, J.; Zhang, B. Photocatalytic CO2 Reduction Coupled with Alcohol Oxidation over Porous Carbon Nitride. Catalysts 2022, 12, 672. https://doi.org/10.3390/catal12060672
Qiu C, Wang S, Zuo J, Zhang B. Photocatalytic CO2 Reduction Coupled with Alcohol Oxidation over Porous Carbon Nitride. Catalysts. 2022; 12(6):672. https://doi.org/10.3390/catal12060672
Chicago/Turabian StyleQiu, Chuntian, Shan Wang, Jiandong Zuo, and Bing Zhang. 2022. "Photocatalytic CO2 Reduction Coupled with Alcohol Oxidation over Porous Carbon Nitride" Catalysts 12, no. 6: 672. https://doi.org/10.3390/catal12060672