1. Introduction
The widespread effects of global warming are evident in most parts of the world in the form of shifting temperature and weather patterns, chiefly due to callous fossil fuel use. As a respite to such adverse effects and in our fight to reduce our carbon footprint, the adoption of powerful, sustainable fuel options such as biofuel can be of tremendous support. This approach has led to several studies in ecologically safe fuel options both in on-road and off-road transport systems. Moreover, recent advances in aviation biofuel engineering have made promising outcomes, striding us closer to a sustainable future [
1]. Over the years, there have been increasing efforts in bioethanol production and blending all over the world, especially in the U.S. and Brazil, as reported by the Alternative Fuel Data Centre of the U.S. Department of Energy. The availability of surplus produce and resources have been the reasons for the rise in bioethanol production levels (
Figure 1a).
Although the U.S. has been a major contributor to the global bioethanol production, the ASD reports have estimated that a very small percentage of this bioethanol comes from cellulosic biomasses, while the major substrate contributors are mainly starch-based [
2] (
Figure 1b).
Biofuel production was originally initiated by utilizing edible feedstock, mainly sugarcane or corn, leading to first-generation biofuel. However, the threatened food security and nonviability associated with this option has led to widespread scouring of strategies that can utilize surplus lignocellulosic biomass as a carbon source, resulting in the development of second-generation biofuel. The biomass is first pre-treated with any of the several chemical, physical or biological methodologies to increase the cellulose availability before being subjected to saccharification. The saccharification is a critical step and a significant barrier in determining the overall cost and productivity of the process, as the amount of sugar released in this step will dictate the ethanol productivity in the subsequent steps of bioethanol production. Saccharification primarily employs lignocellulosic-biomass-degrading enzymes such as cellulase to convert cellulose to simple sugars. Different strategies have been tested to improve this hydrolysis rate during saccharification, which includes high-efficiency wild type screening, site directed mutagenesis, protoplast fusion, cloning, heterologous expression, protein engineering, metabolic engineering, over expression, etc. [
3]. Additionally, a wild strain with a potent hydrolytic capacity can be a boon toward improving saccharification yield as well as enabling easy biomass disposal. Cellulases form the crux of the enzymatic cocktail used for saccharification. They are produced by various microbial species, especially some predominant fungal species such as
Trichoderma.
Cellulase production through fungal cultivation can be performed either by solid state fermentation (SSF) or submerged state fermentation (SmF). Economically, SSF is a more favorable option for cellulase production as opposed to SmF [
4]. Although, regardless of the choice of cultivation strategy, the major percentage (~50%) of cost borne in the production of cellulases is in the choice of carbon source [
5], while utility and operation costs form the rest of the cost of production. However, when coupled with immobilization, SmF can be adapted to be a more powerful economical approach. Any process utilizing enzymes opens a possibility of improving the feasibility of the process by incorporating immobilization. The increased robustness, enzyme recovery, reuse and ability to recycle poses a heavy advantage over all other existing methodologies [
6]. Additionally, enzyme immobilization in industries have lately become a predominant practice mainly because the facility to recycle enzymes several times will definitely bring down the cost of enzyme production by a significant margin, ultimately making biofuel production, and blending them in our everyday fuel, a tangible reality [
7].
The challenges associated with the commercial scale-up of biorefineries are numerous, owing to the uncertain outcomes linked with them, unlike the established fossil fuel practices. The continuous biofuel production industry is, in itself, yet to gain industrial feasibility due to many factors associated with the ambiguity of process alternatives and resource choice. Although there have been a few success stories such as that of Novamont’s 30kt 1-4 butanediol plant and LCY Biosceinces’s 30kt succinic acid plant, there have been equally many cases of setbacks. Examples of them include KiOR, the first cellulosic transport fuel company which went bankrupt in 2014, and Abengoa, which was forced to resell its biofuel production facilities due to bankruptcy during late 2015. Furthermore, Mascoma cancelled its plans to build its USD 232 million proprietary, one-pot, bioethanol-production-from-wood-chips plant due to major stakeholders withdrawing their financial support [
8].
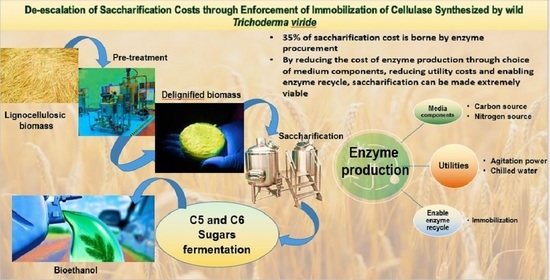
4. Discussion
The economic uncertainty of cellulosic ethanol makes its viability an ambiguous predicament. Therefore, it is of great importance to make it as feasible as possible for it to be a tangible reality. The synthesis of cellulosic ethanol primarily involves three stages. They are: pretreatment of lignocellulosic biomass; saccharification of complex cellulosic polymers to simple sugars; and fermentation of the sugars to bioethanol. Saccharification costs about a third of the total ethanol production and hence, this paper targets different approaches that can effectively reduce this cost. Our study has indicated that two major factors take up a major fraction of operating expenditures in the enzyme production process. They are: media raw materials, and utilities, at 30–50% and 30–40%, respectively. The direct and indirect expenses associated with a large-scale process in addition to the annual opex are detailed in
Table 3,
Table 4,
Table 5 and
Table 6. It is difficult to accurately estimate the total direct cost of the process due to the uncertainty in process costing rates along the years and thus, the data presented have been on the higher side. On the other hand, the operating expenses can be more certainly estimated with the current pricing trends in utilities, labor, media raw materials, facility overhead, lab QC/QA and annual depreciation %. The total direct fixed capital, direct costs contribute to 54.4% of the total amount, whilst indirect costs account for 32.6%.
The major media components that contribute to the operating cost fraction are carbon and nitrogen sources. In our study, among the seven different production media, modified wheat bran media with glucose as a simple carbon source, along with wheat bran and cellulose, which acted as both a complex carbon source and inducer, and further supplemented organic and inorganic nitrogen elements and salts was found to be optimal. This medium not only supported good mycelial growth and propagation (60 PCV) but also good cellulase productivity levels, with values up to 7.4 U/mL.
While PDB, the basal medium, supports good growth with a specific growth rate of 0.6 h
−1 and PCV of 58 at the 24th hour, it is not optimal for cellulase production. Hence, the media components were designed to independently modify the needs of
Trichoderma viride for biomass growth as well as cellulase production. In addition to the simple carbon source, complex carbon and nitrogen sources such as wheat bran and microcrystalline cellulose were added to circumvent catabolite repression issues. Since cellulose is reported to have a deleterious influence at high concentrations (>3%) due to its irreversible adsorption with cellulases, it has been used in combination with wheat bran (1:1) at low concentrations (1%). Furthermore, studies have attributed low cellulose levels to enhanced biomass growth and cellulase activity [
14]. Additionally, corn steep liquor (CSL) has been reported to support high cellulase production in
Trichoderma reesei, which is why it was incorporated in the production media [
15]. Thus, media design studies led to the formulation of a wheat-bran-based media incorporating wheat bran, microcrystalline cellulose, corn steep liquor and glucose for high cell density and enzyme production (
Table 1). Furthermore, when dextrose was used as the sole carbon source, the fungal growth and reproduction thrived but with very little cellulase production due to carbon catabolite repression (CCR), as reported in the referenced study [
16]. CCR is avoided by using a strategy of incorporating complex carbon sources along with glucose. Such an integration effectively supported early fungal growth and propagation. The complex carbon sources, wheat bran and microcrystalline cellulose, contributed heavily toward cellulase induction and cell biomass sustenance in the later phase of fermentation when the cells were starved of glucose, an easily utilizable carbon source. The complex carbon and nitrogen sources in the media take precedence during this nutrient-deprived phase, which forces the
Trichoderma viride cells to produce cellulases in order to utilize the complex nutrients for their survival. The modified wheat bran 3 medium showed promising outcomes with the inclusion of glucose as well as wheat bran and cellulose. Corn steep liquor, ammonium sulphate and soybean meal have prolifically supported the cellulase formation as well as cell metabolism during the production phase. Hence, an optimal production medium was formulated (Modified WB media 3—
Table 1).
When a larger scale of production is considered, glucose, an easily utilizable carbon source, is priced at more than 30% of the total media cost. However, due to its critical role in rapid biomass growth and proliferation, it cannot be avoided, and instead we can look into alternate ways to obtain this glucose. The initial glucose can be added externally or it can be procured from a fraction of the already saccharified lignocellulosic biomass slurry that can serve as an effective starting carbon source for the fungal growth. In any case, this cost is fixed and cannot be changed much. Alternatively, other, costlier media components such as microcrystalline cellulose and yeast extract can be supplanted with industrial-grade equivalents. Many studies have proposed the use of lignocellulosic waste and other industrial waste by-products for cellulase production, but the productivity levels reported using such substrates have been subpar with the industrial requirements. Some of such reported agro waste substrates are coconut shell, grape marc, fruit peel, sugarcane bagasse, saw dust, etc. [
17]. Thus, they must be used in an amalgamation with other expensive components but at lower ratios. Although a waste-to-value-added product seems to be a very attractive approach, it is very rarely the case when a real-time process scale-up is performed. High cellulase productivity levels have been reported using agro wastes, such as corn cob, as both carbon and nitrogen sources [
18], yet the cost of these processed agro wastes reflect a different setting in the pursuit of an economically viable cellulase production strategy. The lignocellulosic waste is, in itself, very inexpensive but cannot be used in its native form and thus requires a range of pretreatments before it can be used as a substrate for an enzymatic hydrolysis. These steps compound the final cost of the lignocellulosic waste, influencing the operating costs directly. Hence, very limited substitutes are available which can be deemed profitable when the process is scaled up. Thus, this study calls for additional analysis to address this concern. Similarly, cheap nitrogen sources such as industrial grade nitrogen sulphate and corn steep liquor can be used in media instead of yeast extract. Moreover, ammonium sulphate and CSL are easily obtainable forms of readily available sources of not only free nitrogen but also sugar, salts and other micronutrients for microbial populations and thus is a more preferred nitrogen source. Its supplement can help maintain 300–400 ppm of freely available nitrogen in the media at all times, helping cells to assimilate sufficient nitrogen for adequate cellulase production [
16].
This inclusion of nonedible agro wastes and industrial by-products will prove to be a boon to many industries, which will encourage the establishment of cogenerative facilities all over the world. Cogenerative biofuel production can be a revolutionary solution to the growing utility and transportation costs.
Further, to reduce the overall utility costs, DSP operations can be eliminated to an extent wherein purity of the end-product cellulase can be compromised in industrial settings where the cogeneration of bioethanol forms an added advantage to the already existing processes, saving a whopping 57.4% in overall operation costs. Moreover,
Trichoderma viride is a well-known entophytic symbiont that has been proven to enhance the nutritional value of food, restore soil fertility, strengthen plant defense systems toward both biotic and abiotic stress and increase the plant productivity through colonization of the root system, such as in the case of rice, tomato, ghost pepper, cabbage, etc. [
19,
20,
21,
22]. Hence, the fungal biomass can be separated and disposed of easily and effectively. In industrial environments, such as sugar mills where large amount of nonedible lignocellulosic biomass is generated every day, production of bioethanol using in-house-produced cellulases can generate surplus income with minimal investment in equipment costs and easy disposal of microbial waste. In such a process setting, the cost of carbohydrates will ultimately surpass the DSP costs, but cannot be clearly defined due to the generation of coproduct credits and energy requirements of the process through recycling or other side-stream processes in the highly integrated fermentation biorefinery [
23]. Further modelling and analysis of cellulase production systems by Johnson has proven that integrated cellulase production systems lowers the ultimate cellulosic bioethanol cost when compared to that of off-site systems [
24].
Additionally, the agitation power costs and chilled water costs can also be minimized using alternate microbial sources. Pelleted fungal cultures make excellent enzyme producers whilst mimicking bacterial culture rheology. Alternatively, bacterial strains such as
Bacillus cereus,
Bacillus subtilis and
Bacillus thuringiensis have shown good cellulase productivities using agro waste or CMC as substrates. However, their productivity levels cannot match that of fungal strains [
25]. Furthermore, the culture cultivation and enzyme separation in the case of fungal producers are much easier than in bacterial production systems. However, unlike bacterial fermentation, fungal processes run for a longer period, due to which the utilities such as chilled water, aeration and agitation are needed for a longer duration, necessitating its availability. Although utilities comprise a major fraction of operation costs, they cannot be compromised and thus form a large percentage of the operating expenditure. Hence, future studies must focus on developing better cellulase-producing bacterial strains as utilities consumed by one fungal cultivation can be used to run almost 4–5 bacterial batches, given their current cellulase productivities.
Apart from regulation of microbial strain and media component selection, increasing the shelf life of enzymes can work wonders toward saccharification costs. Enzyme purchase costs alone account for more than 35% of saccharification costs and thus represent a significant fraction when lignocellulosic hydrolysis economics are considered [
7,
26]. Additionally, if the enzyme shelf life can be extended or if it can be recovered and recycled after every enzymatic process, the saccharification costs can be significantly reduced. Immobilization is one such powerful strategy to ensure smoother enzyme recovery. The major bottleneck lies in the effective separation of the immobilized enzymes from the saccharification slurry. Iron-oxide magnetic nanoparticles are the best option to overcome this issue. We were successfully able to immobilize cellulases from the wild
Trichoderma viride on to IOMNPs using EDC (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide) functionalization with a binding efficiency of 83.5%, in addition to which the immobilized enzymes retained more than 55% of total cellulase activity at the end of the fifth hydrolytic cycle. These IOMNP-coupled cellulases can easily bind to lignocellulosic biomass and are easy to separate. At such high binding and stable activity rates, cellulase immobilization will effectively reduce the annual enzyme procurement costs as well as facilitate easier cellulase separation from the saccharification mixture.
The cost breakdown of the production of free cellulases versus iron-oxide magnetic nanoparticle immobilized cellulases places the immobilization cost to three times that of the former. However, on comparison of cost vs. recycling, IOMNP-immobilized cellulases exhibited an average of 90% hydrolytic efficiency at the end of third hydrolytic cycle and thus reflect as a valuable alternative to the free enzymatic saccharification process and hence forms a point of breakeven. Hence, IOMNP-immobilization of cellulases can drastically alter the saccharification economics, resulting in a more profitable approach. Future studies can work on the extension of recycling immobilized enzyme systems and can be the best possibility in certain reduction of saccharification costs. Further, a mixed system of free enzymes and immobilized enzymes can also be used to compensate for the reduced hydrolysis rates in immobilized systems. This will ensure higher saccharification efficiency and have a positive effect on ultimate saccharification yields.
Thus, in order to bring down the cost of cellulosic bioethanol, the process has to be optimized at every point. The adoption of best yielding practices at every step of the process is the only way through which we can attain the blending goals of bioethanol in everyday fuel. Hence, this work is of great significance in reflecting the enormous implications of lab-scale cellulase studies on the path for cleaner fuel.
5. Materials and Methods
5.1. Experimental Data
All the experiments were carried out with wild the Trichoderma viride strain, kindly provided by Jawaharlal Nehru University, New Delhi. The medium was designed and the batch fermentation was performed with the media that supported the highest productivity.
Seven different media were tested for their ability to support growth and cellulase formation in Trichoderma viride in 3 litres New Brunswick CelliGen 115 STR, Eppendorf, Germany (Stirred tank reactor) and further scaled up in 7 litres New Brunswick BIOFLO® 415 STR, Eppendorf, Germany, in the semisolid submerged cultivation method.
The primary focus was on high biomass build-up and its sustainability during the stationary phase because stationary phase conditions favour mycelial conservation along with high cellulase productivity [
16]. Wheat bran, microcrystalline cellulose, lactose and glucose were tested for their competence in being both a carbon source and inducer in various permutations. When dextrose was used as the sole carbon source, the fungal growth and reproduction thrived but with very little cellulase production due to carbon catabolite repression as reported in the referenced study [
27]. Carbon catabolite repression was avoided using a strategy of incorporating complex carbon sources along with glucose. Such an integration effectively supported early fungal growth and propagation. The complex carbon source, wheat bran and microcrystalline cellulose, contributed heavily toward cellulase induction and cell biomass sustenance in the later phase of fermentation when the cells were starved of glucose, an easily utilizable carbon source. The complex carbon and nitrogen sources in the media take precedence during this nutrient-deprived phase, which forces the
Trichoderma viride cells to produce cellulases in order to utilize the complex nutrients for its survival. The modified wheat bran 3 medium showed promising outcomes with the inclusion of glucose as well as wheat bran and cellulose. Corn steep liquor, ammonium sulphate and soybean meal have prolifically supported the cellulase formation as well as cell metabolism during the production phase.
The temperature for the process was maintained at 28 °C and the agitation was controlled based on increasing biomass and DO (dissolved oxygen) levels (500 to 1000 rpm). The DO levels were monitored using DO probes (Mettler Toledo, OH, USA, InPro 6850i) continuously, and at any given instant at least a 30% DO level was maintained in the vessel (2 vvm air) through compressor (Sthiraa, Chennai, India, oil free compressor, Model: EA 750). The pH was controlled (Mettler Toledo, 405-DPAS-SC-K8S/225) and maintained at 7.0 automatically, using 2 N H2SO4 and 2 N NaOH.
5.2. Inoculum Preparation
The inoculum for the process was cultivated in the three stage seed method from spore glycerol stocks of Trichoderma viride. About 100 µL of spore glycerol stocks of Trichoderma viride were inoculated in PDA (Potato dextrose agar) and grown at 30 °C for 7 days until a deep green mature spore mat forms. The spores were harvested using 0.9% sterile NaCl which were inoculated in PDB (Potato dextrose broth) at a concentration of 1 × 108 spores/mL. The germinated spore culture was further sub-cultured three times at incremental volumes to generate the final inoculum of 20% of production media volume. The reason for the subculture was to generate a full, log-growth phased inoculum that could adapt to the production media with the least amount of lag phase as possible.
5.3. Biomass Estimation
The fungal biomass was estimated using packed cell volume (PCV) with a 5% correlation between the dry cell weights of the fungal culture to PCV. The fermentation broth is centrifuged at 3400×
g rpm for 5 min and the pellet volume is used to determine PCV (Equation (1)).
5.4. Determination of Cellulase Activity
The residual sugar was estimated through glucose oxidase and peroxidase (GOD-POD) Assay using Liquizone Glucose-MR (GOD-POD) kit (Medsource Ozone Biomedicals Pvt. Ltd., Haryana, India). The collected sample was centrifuged at 8000 rpm for 5 min at 4 °C. The supernatant was then used for residual glucose determination [
28].
The total cellulase activity, exoglucanase activity, endoglucanase activity and cellobiase activity was measured using standard National Renewable Energy Laboratory (NREL) protocols. The protein concentrations were measured using Bradford Assay [
29] (SRL, Mumbai, India).
5.5. Preparation of Iron-Oxide Magnetic Nanoparticles (IOMNPs)
The IOMNPs were prepared by coprecipitation of ferric and ferrous ions in a 2:1 ratio under vigorous stirring under alkali condition (pH 8) for 2 h. The precipitate was washed three times with water and once with anhydrous ethanol and a magnet was used to separate and test the magnetic properties of the IOMNPs.
5.6. EDC Functionalization of IOMNPs and Cellulase Immobilization
50 mg of IOMNPs was suspended in 5 mL of 8 mg/mL EDC (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide) and sonicated for 3 min followed by cooling of the suspension to 4 °C. The enzyme was added and the mixture was sonicated for 3 min at 4 °C twice with a one hour interval, and finally the suspension was heated to 25 °C. The cellulase-conjugated nanoparticle was separated and washed with distilled water two times and stored in PBS (pH 7.0) [
10].
Binding efficiency and relative activity have been used to check for the competence of binding between cellulase and IOMNPs (Equations (2) and (3)). Bradford assay was used to determine the amount of enzyme bound.
5.7. Optimization of pH and Temperature for Cellulase-Bound IOMNPs’ Stability
Different pH conditions were tried to check for the stability of the cellulase functionality after immobilization onto IOMNPs. Different pH variations (pH 3.0, 4.0, 5.0 and 6.0) were maintained using citrate buffer while phosphate buffer saline was used for pH 7.0.
Temperature variations between 25 °C to 80 °C were performed to check the stability of cellulose-immobilized nanoparticles using water bath.
All the chemicals for immobilization experiments were procured from Merck, Germany.
5.8. Activity Preservation
Cellulase assay (FPase Assay) was performed to check for the activity of the cellulase-conjugated IOMNPs. They were separated after every assay and set for cellulase assay with fresh substrate to check for subsequent activity changes with each cycle.
5.9. Capital Expenditure (CAPEX)
The total direct fixed capital of the cellulase production includes direct costs (equipments, process piping, instrumentation, insulation, electrical, buildings, yard improvement and auxiliary facilities), indirect costs (engineering and construction) and other costs (contractor fee and contingency). The total direct costs were estimated using the proportionality factors obtained from bioprocess design and economics [
30]. The indirect costs of engineering were estimated to be 0.25 times the direct cost, while construction costs were 0.35 times that of direct costs. Further, contractor fees and contingency costs were estimated to be 0.05 ∗ (direct cost + indirect cost) and 0.10 ∗ (direct cost + indirect cost), respectively.
Table 2 represents the equipment costs to be invested while establishing a cellulase production plant as per
Figure 3. This is a one-time requisite capital investment which should be taken into account when the overall economics of the process is considered. The equipment costs were estimated using Equation (13), where the cost-to-capacity method was used and the scale factor for each piece of equipment was taken from the literature. The scale factor for installation, piping, instrumentation, insulation, electrical, building development, yard improvement and auxiliary facilities are 0.5, 0.4, 0.35, 0.03, 0.15, 0.45, 0.15 and 0.5, respectively (
Table 6) [
4]. Furthermore, the six-tenths rule was used to determine the equipment costs from pre-existing data (Equation (4)).
Additionally, the annual maintenance and depreciation charges making up to 15% of the total fixed assets cost were considered.
5.10. Operating Expenses (OPEX)
5.10.1. Medium Cost
The medium component cost was estimated for a process of 1000 m3 (100 m3 × 10 bioreactors) scale batch process. Although the media components used for the batch operation were laboratory grade, the media component costs were calculated through the market prices obtained from e-commerce platforms and business intelligence. The component costs were extrapolated from the market price and calculated in accordance to the amount used in the optimized media.
On cost analysis of the production media, carbon source (Glucose and Avicel) formed a major percentage (more than 50%) of the media costs, with yeast extract and soybean meal contributing a significant part of the rest. Although, in a large-scale industrial batch, bulk lignocellulosic biomass and industrial grade glucose/invert sugar will be used, making them a critical upstream cost contributor. The inclusion of avicel and cheaper lignocellulosic biomass (wheat bran) to obtain a higher cellulase production through autoinduction in a wild strain can be economically welcoming when the process is scaled up. Furthermore, experimental studies using these media have supported higher biomass and enhanced productivity.
In order to reduce the cost of media components without compromising productivity, industrial grade raw materials such as delignified sugarcane bagasse and wheat straw were proposed as sources of cellulose while corn steep liquor, yeast and corn meal are more potent and inexpensive nitrogen sources. Ultimately, this enabled the reduction of media costs by almost 50%.
5.10.2. Utility Costs
The cellulase production process is a 72 h process. When annual revenue of such a process is calculated, the cleaning period and non-operational period are to be taken into consideration. It is assumed that in a year, 20 days are non-operational and used for maintenance purposes, and an additional 24 h are required to clean and prepare the equipment for the subsequent batches. The utility costs were calculated for the operational period of the process.
5.11. Aeration and Agitation
Air supply, along with agitation, is crucial in maintaining the DO levels in the bioreactor, especially in fungal fermentation. At high biomass concentrations, the mycelial growth network tends to limit the oxygen transfer within the bioreactor, necessitating a continuous and efficient oxygen distribution system. The air supply was maintained in a large-scale bioreactor between 0.5–0.6 vvm. The cost estimation of aeration requirement, compressor power requirement and agitator power requirement per 1000 m
3 batch fermentation was performed using the power consumption and oxygen uptake rate data obtained during the experimentation along with the market pricing for both oxygen and electricity, which was USD 0.52/m
3 and USD 0.126/kWh, respectively. Using these parameters, the overall cost of aeration and power consumption was calculated considering an ideal, single-stage compressor with electrical and mechanical compression losses taken into account using the formulae (Equation (5)) [
4].
where:
PC: Power consumption of compressor, kW;
QAIR: Air volumetric flow rate, L/min;
p1: Inlet absolute pressure of compressor, Pa;
p2: Outlet absolute pressure of compressor, Pa;
ɳc: Assumed efficiency number of compressor = 0.7;
γ: Isoentropic exponent, 1.4 for air.
Power consumption for a 7 L New Brunswick BIOFLO
® 415 continuously stirred tank reactor with three Rushton turbine impellers was calculated using the formulae for power consumption for ungassed stirring (Equation (6)).
where:
P: Power consumption of ungassed stirring, kW;
Np: Power number, 5.2 (Rushton impeller in turbulent regime, according to the Reynolds number);
ρb: Broth density, kg/m3;
N: Number of impeller blades;
g: Gravitational acceleration, m/s2;
Where fc is given by Equation (7).
DR: Diameter of the reactor = 14.6 cm;
Di: Impeller Diameter = 5.9 cm;
HL: Height of the liquid = 33.6 cm;
‘∗’: Bioreactor measurements used in the experiment.
Power consumption in gassed, stirred systems is calculated using the following equation (Equations (8) and (9)).
where:
P: Power consumption of ungassed stirring, kW;
QG: Total gas volumetric flow rate, m3/s;
ɳS: Energy efficiency, 0.65;
N: Stirrer speed.
5.12. Temperature Maintainenace
The fermentation duration of maximum cellulase production was 72 h, during which it was imperative to maintain the operational conditions of temperature, pH and oxygen levels in the fermenter. The optimal growth temperature of Trichoderma viride was 28–30 °C, which is lower than the ambient temperature, thus increasing the chilled water requirement during the bioprocess. As the chiller works to remove the metabolic heat generated by cells and the heat given out by the agitator, to maintain the temperature in the vessel, power consumption to remove the heat from cooling water proportionally increases.
Apart from the chilled water used for temperature maintenance, an integral part of every bioprocess, the sterilization of production media and bioreactor also consumes steam and cooling water. Steam is utilized by the reactor during the in situ sterilization process to heat the media to 121 °C and to generate pressure in the reactor. Further, forced temperature drop of the media to growth temperature (28–30 °C) involves cool water circulation in the reactor. Thus, the total cost analysis of the process takes into account the steam costs, chilled water costs and cooling water costs. Assuming negligible environmental losses and that all the heat is transferred to the cooling water, the cost of cooling was calculated.
The total heat generated in the agitator (
Es) was calculated by integrating the gassed stirring power consumption over the time taken to reach maximum enzyme activity. The total energy consumed while stirring is given by Equation (10).
The total metabolic heat (
EM) generated was similarly calculated by integrating the metabolic heat release rate over the time taken to reach maximum enzyme activity. The total metabolic heat generated in a bioprocess is given by Equations (11) and (12).
where:
QHEAT: Metabolic heat release, kJ/h;
K: Constant of proportionality, 0.50 kJ/mmol O2;
OUR: Oxygen uptake rate, mmol O2/(Lh);
VR: Volume of broth in the reactor.
The cost of cooling was calculated by Equation (13).
where:
5.13. Downstream Processing
Downstream-processing operating costs take more than 50% of the utility costs when the separation and purification of enzyme is needed and is the most important factor contributing to the production cost. DSP operations and equipment such as centrifugation, concentrators, freeze dryers and sterilizing drums are a critical necessity for purifying and concentrating the enzyme to an acceptable purity which can further be marketed. The cost estimate for the DSP operations were calculated from data obtained from the fiscal study by Zhuang et al. on cellulase production for bioethanol and the prices were adjusted to the current inflation rates for a 1000 m3 scale process.
5.14. Immobilization Costs
The cost of immobilization was estimated using the price of iron-oxide nanoparticles taken from commercial business websites including the cost associated with the immobilization process.