The Role of Steps on Silver Nanoparticles in Electrocatalytic Oxygen Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Properties of Ag(322)
2.2. Adsorption of Atomic and Molecular Oxygen on Ag(322)
2.3. Dissociation of O2 on Ag(322)
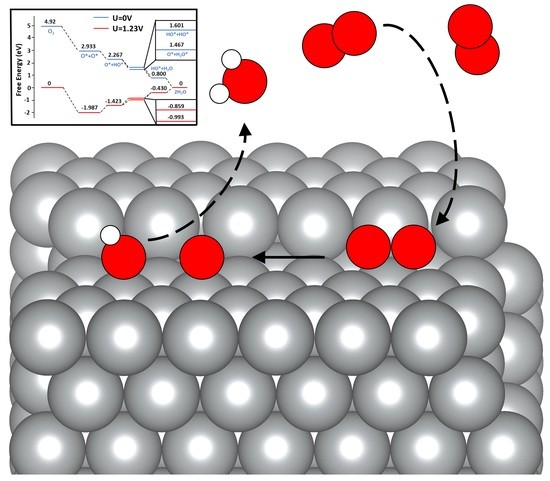
2.4. Electrocatalytic ORR Performance on Ag(322)
3. Computational Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakenne, A.; Nuttall, W.; Kazantzis, N. Sankey-Diagram-based insights into the hydrogen economy of today. Int. J. Hydrogen Energy 2016, 41, 7744–7753. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage – past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Kushnir, D.; Hansen, T.; Vogl, V.; Åhman, M. Adopting hydrogen direct reduction for the Swedish steel industry: A technological innovation system (TIS) study. J. Clean. Prod. 2020, 242, 118185. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- McDowall, W.; Eames, M. Towards a sustainable hydrogen economy: A multi-criteria sustainability appraisal of competing hydrogen futures. Int. J. Hydrogen Energy 2007, 32, 4611–4626. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Fu, C.; Liu, C.; Li, T.; Zhang, X.; Wang, F.; Yang, J.; Jiang, Y.; Cui, P.; Li, H. DFT calculations: A powerful tool for better understanding of electrocatalytic oxygen reduction reactions on Pt-based metallic catalysts. Comput. Mater. Sci. 2019, 170, 109202. [Google Scholar] [CrossRef]
- Hinsch, J.J.; Liu, J.; Wang, Y. Reinvestigating oxygen adsorption on Ag(111) by using strongly constrained and appropriately normed semi-local density functional with the revised Vydrov van Voorhis van der Waals force correction. J. Chem. Phys. 2021, 155, 234704. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, K.; Zhang, Q.; Wu, H.; Gu, L.; Yao, K.; Shen, Y.; Shao, Y. In-situ synthesis, operation and regeneration of nanoporous silver with high performance toward oxygen reduction reaction. Nano Energy 2019, 58, 69–77. [Google Scholar] [CrossRef]
- Sarapuu, A.; Kibena-Põldsepp, E.; Borghei, M.; Tammeveski, K. Electrocatalysis of oxygen reduction on heteroatom-doped nanocarbons and transition metal–nitrogen–carbon catalysts for alkaline membrane fuel cells. J. Mater. Chem. A 2018, 6, 776–804. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K. Oxygen Reduction Reaction on Silver Catalysts in Alkaline Media: A Minireview. ChemElectroChem 2019, 6, 73–86. [Google Scholar] [CrossRef]
- Kitayev, A.; Zysler, M.; Hardisty, S.; Page, M.; Tal-Gutelmacher, E.; Zitoun, D. Silver Oxygen Reduction Electrocatalyst in Alkaline Medium: Aging and Protective Coating. Energy Technol. 2021, 9, 2100546. [Google Scholar] [CrossRef]
- Nubla, K.; Sandhyarani, N. Ag nanoparticles anchored Ag2WO4 nanorods: An efficient methanol tolerant and durable Pt free electro-catalyst toward oxygen reduction reaction. Electrochim. Acta 2020, 340, 135942. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Merisalu, M.; Matisen, L.; Käärik, M.; Leis, J.; Sammelselg, V.; Aruväli, J.; Kaljuvee, T.; Tammeveski, K. Oxygen Reduction on Silver Nanoparticles Supported on Carbide-Derived Carbons. J. Electrochem. Soc. 2018, 165, F1199–F1205. [Google Scholar] [CrossRef]
- Shi, Y.M.; Zhou, W.; Lu, A.Y.; Fang, W.J.; Lee, Y.H.; Hsu, A.L.; Kim, S.M.; Kim, K.K.; Yang, H.Y.; Li, L.J.; et al. van der Waals Epitaxy of MoS2 Layers Using Graphene As Growth Templates. Nano Lett. 2012, 12, 2784–2791. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Guan, W.; Zhang, L.; Fan, X.; Shi, Z.; Zheng, W. Shape-dependent catalytic activity of oxygen reduction reaction (ORR) on silver nanodecahedra and nanocubes. J. Power Sources 2014, 269, 152–157. [Google Scholar] [CrossRef]
- Jones, T.E.; Rocha, T.C.R.; Knop-Gericke, A.; Stampfl, C.; Schlögl, R.; Piccinin, S. Insights into the Electronic Structure of the Oxygen Species Active in Alkene Epoxidation on Silver. ACS Catal. 2015, 5, 5846–5850. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, J.; Yang, S.; Liu, T.; Tao, K.; Chang, H. A silver wire aerogel promotes hydrogen peroxide reduction for fuel cells and electrochemical sensors. J. Mater. Chem. A 2019, 7, 11497–11505. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, F.; Liu, D.; Xia, Z. Electrochemical Oxygen Reduction Reaction in Alkaline Solution at a Low Overpotential on (220)-Textured Ag Surface. ACS Appl. Energy Mater. 2018, 1, 4385–4394. [Google Scholar] [CrossRef]
- White, J.J.; Liu, J.; Hinsch, J.J.; Wang, Y. Theoretical understanding of the properties of stepped iron surfaces with van der Waals interaction corrections. Phys. Chem. Chem. Phys. 2021, 23, 2649–2657. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Su, G.; Ferri, N.; Liu, W.; Tkatchenko, A. Tuning the work function of stepped metal surfaces by adsorption of organic molecules. J. Phys. Condens. Matter 2017, 29, 204001. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; White, M.G.; Liu, P. Oxygen Reduction Reaction on Ag(111) in Alkaline Solution: A Combined Density Functional Theory and Kinetic Monte Carlo Study. ChemCatChem 2018, 10, 540–549. [Google Scholar] [CrossRef]
- Andryushechkin, B.V.; Shevlyuga, V.M.; Pavlova, T.V.; Zhidomirov, G.M.; Eltsov, K.N. Adsorption of molecular oxygen on the Ag(111) surface: A combined temperature-programmed desorption and scanning tunneling microscopy study. J. Chem. Phys. 2018, 148, 244702. [Google Scholar] [CrossRef]
- Raukema, A.; Butler, D.A.; Box, F.M.A.; Kleyn, A.W. Dissociative and non-dissociative sticking of O2 at the Ag(111) surface. Surf. Sci. 1995, 347, 151–168. [Google Scholar] [CrossRef]
- Xu, Y.; Greeley, J.; Mavrikakis, M. Effect of Subsurface Oxygen on the Reactivity of the Ag(111) Surface. J. Am. Chem. Soc. 2005, 127, 12823–12827. [Google Scholar] [CrossRef]
- Montemore, M.M.; Spronsen, M.A.V.; Madix, R.J.; Friend, C.M. O2 Activation by Metal Surfaces: Implications for Bonding and Reactivity on Heterogeneous Catalysts. Chem. Rev. 2018, 118, 2816–2862. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zeng, X.C. Unraveling Oxygen Evolution in Li-Rich Oxides: A Unified Modeling of the Intermediate Peroxo/Superoxo-like Dimers. J. Am. Chem. Soc. 2019, 141, 10751–10759. [Google Scholar] [CrossRef]
- Wasileski, S.A.; Janik, M.J. A first-principles study of molecular oxygen dissociation at an electrode surface: A comparison of potential variation and coadsorption effects. Phys. Chem. Chem. Phys. 2008, 10, 3613–3627. [Google Scholar] [CrossRef]
- Otani, M. Electrocatalysis: Theory and experiment at the interface. Phys. Chem. Chem. Phys. 2008, 10, 3607–3608. [Google Scholar]
- Polo, V.; Kraka, E.; Cremer, D. Electron correlation and the self-interaction error of density functional theory. Mol. Phys. 2002, 100, 1771–1790. [Google Scholar] [CrossRef]
- Bao, J.L.; Gagliardi, L.; Truhlar, D.G. Self-Interaction Error in Density Functional Theory: An Appraisal. J. Phys. Chem. Lett. 2018, 9, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Huang, Z.-Q.; Zhang, Y.; Chang, C.-R. Trends in water-promoted oxygen dissociation on the transition metal surfaces from first principles. Phys. Chem. Chem. Phys. 2017, 19, 2364–2371. [Google Scholar] [CrossRef]
- Sargeant, E.; Illas, F.; Rodríguez, P.; Calle-Vallejo, F. Importance of the gas-phase error correction for O2 when using DFT to model the oxygen reduction and evolution reactions. J. Electroanal. Chem. 2021, 896, 115178–115184. [Google Scholar] [CrossRef]
- Tesch, R.; Kowalski, P.M.; Eikerling, M.H. Properties of the Pt(111)/electrolyte electrochemical interface studied with a hybrid DFT–solvation approach. J. Phys. Condens. Matter 2021, 33, 1. [Google Scholar] [CrossRef]
- Sun, J.; Ruzsinszky, A.; Perdew, J.P. Strongly Constrained and Appropriately Normed Semilocal Density Functional. Phys. Rev. Lett. 2015, 115, 036402–036408. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Yang, Z.-H.; Perdew, J.P.; Sun, J. Versatile van der Waals Density Functional Based on a Meta-Generalized Gradient Approximation. Phys. Rev. X 2016, 6, 041005. [Google Scholar] [CrossRef] [Green Version]
- Sharkas, K.; Wagle, K.; Santra, B.; Akter, S.; Zope, R.R.; Baruah, T.; Jackson, K.A.; Perdew, J.P.; Peralta, J.E. Self-interaction error overbinds water clusters but cancels in structural energy differences. Proc. Natl. Acad. Sci. USA 2020, 117, 11283–11288. [Google Scholar] [CrossRef]
- Kamrani Moghaddam, L.; Ramezani Paschepari, S.; Zaimy, M.A.; Abdalaian, A.; Jebali, A. The inhibition of epidermal growth factor receptor signaling by hexagonal selenium nanoparticles modified by SiRNA. Cancer Gene Ther. 2016, 23, 321–326. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Na My, O.; Xuan Huynh, N.T.; Thi, P.T.; Chihaia, V.; Ngoc Son, D. Mechanism and activity of the oxygen reduction reaction on WTe2 transition metal dichalcogenide with Te vacancy. RSC Adv. 2020, 10, 8460–8469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linge, J.M.; Erikson, H.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Oxygen reduction on silver catalysts electrodeposited on various nanocarbon supports. SN Appl. Sci. 2021, 3, 263. [Google Scholar] [CrossRef]
- Li, B.; Gao, W.; Jiang, Q. Electronic and geometric determinants of adsorption: Fundamentals and applications. J. Phys. Conf. Energy 2021, 3, 022001. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Tymoczko, J.; Colic, V.; Vu, Q.H.; Pohl, M.D.; Morgenstern, K.; Loffreda, D.; Sautet, P.; Schuhmann, W.; Bandarenka, A.S. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science 2015, 350, 185–189. [Google Scholar] [CrossRef]
- Nanba, Y.; Koyama, M. An Element-Based Generalized Coordination Number for Predicting the Oxygen Binding Energy on Pt3M (M = Co, Ni, or Cu) Alloy Nanoparticles. ACS Omega 2021, 6, 3218–3226. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; An, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Kozlova, J.; Aruväli, J.; Sammelselg, V.; Tammeveski, K. Oxygen reduction on electrodeposited silver catalysts in alkaline solution. J. Solid State Electrochem. 2018, 22, 81–89. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y. Theory-experimental gap. In Multiscale Modeling of Electrochemical Reactions and Processes; Wang, Y., Ed.; AIP Publishing: Melville, NY, USA, 2021; pp. 1-1–1-14. [Google Scholar]
- Wang, Y. Numerical simulation of electrified solid-liquid interface. In Multiscale Modeling of Electrochemical Reactions and Processes; Wang, Y., Ed.; AIP Publishing: Melville, NY, USA, 2021; pp. 3-1–3-18. [Google Scholar]
- Wang, Y.; Liu, X.; Liu, J.; Al-Mamun, M.; Liew, A.W.; Yin, H.; Wen, W.; Zhong, Y.L.; Liu, P.; Zhao, H. Electrolyte Effect on Electrocatalytic Hydrogen Evolution Performance of One-Dimensional Cobalt–Dithiolene Metal–Organic Frameworks: A Theoretical Perspective. ACS Appl. Energy Mater. 2018, 1, 1688–1694. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5467. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy path. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef] [Green Version]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspective. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]






| Absorbate | Ads Site | Eads (eV) | dO-Ag (Å) | vO-O (cm−1) | REF | ||
|---|---|---|---|---|---|---|---|
| 322 | O | SH | −4.40 | 2.24 | n/a | 0 | This work |
| H | −3.02 | 2.13 | n/a | 0 | |||
| O2 | fSH | −0.70 | 2.37 | 668 | 0 | ||
| bSH | −0.27 | 2.36 | 762 | 0 | |||
| 111 | O | fcc | −4.05 | 2.10 | n/a | 0 | [8] |
| O2 | fH | −0.38 | 2.22 | 827 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinsch, J.J.; Liu, J.; White, J.J.; Wang, Y. The Role of Steps on Silver Nanoparticles in Electrocatalytic Oxygen Reduction. Catalysts 2022, 12, 576. https://doi.org/10.3390/catal12060576
Hinsch JJ, Liu J, White JJ, Wang Y. The Role of Steps on Silver Nanoparticles in Electrocatalytic Oxygen Reduction. Catalysts. 2022; 12(6):576. https://doi.org/10.3390/catal12060576
Chicago/Turabian StyleHinsch, Jack Jon, Junxian Liu, Jessica Jein White, and Yun Wang. 2022. "The Role of Steps on Silver Nanoparticles in Electrocatalytic Oxygen Reduction" Catalysts 12, no. 6: 576. https://doi.org/10.3390/catal12060576