Electrodeposition of a Li-Al Layered Double Hydroxide (LDH) on a Ball-like Aluminum Lathe Waste Strips in Structured Catalytic Applications: Preparation and Characterization of Ni-Based LDH Catalysts for Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrodeposition and Calcination of Li-Al-CO3 LDH Thin Film/Characterization of Ni-Based Catalyst on Calcined LDH Support
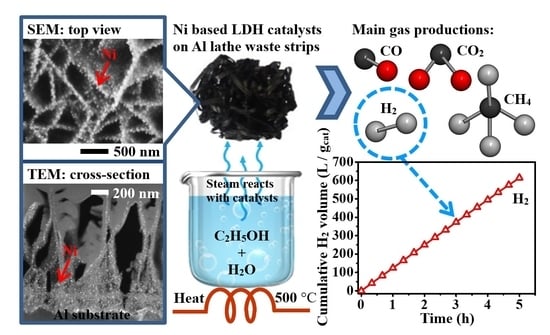
2.2. Use of the Structured Catalyst in Ethanol Steam Reforming (ESR) Reaction
2.3. Ni-Based Catalysts on Calcined LDH Supports: Comparison between NaOH and NaHCO3 Titration in Preparation of the Catalysts
2.4. Mechanism of Carbon (Coke) Formation in Ethanol Steam Reforming (ESR) Reaction
3. Materials and Methods
3.1. Preparation and Calcination of Li-Al LDH Thin Film
3.2. Preparation of Ni-Based Catalyst on Calcined LDH Thin Film
3.3. Tests of Catalysts in Ethanol Stream Reforming (ESR)
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benito, M.; Sanz, J.L.; Isabel, R.; Padilla, R.; Arjona, R.; Daza, L. Bio-ethanol steam reforming: Insights on the mechanism for hydrogen production. J. Power Sources 2005, 151, 11–17. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Chang, W.-R.; Hwang, J.-J.; Wu, W. Environmental impact and sustainability study on biofuels for transportation applications. Renew. Sustain. Energy Rev. 2017, 67, 277–288. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Hourng, L.-W. Effects of magnetic field and pulse potential on hydrogen production via water electrolysis. Int. J. Energy Res. 2014, 38, 106–116. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Wang, X.-F. G-C3N4/Ti3C2Tx (Mxenes) composite with oxidized surface groups for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- Lin, M.-C.; Uan, J.-Y.; Tsai, T.-C. Fabrication of AlLi and Al2Li3/Al4Li9 intermetallic compounds by molten salt electrolysis and their application for hydrogen generation from water. Int. J. Hydrogen Energy 2012, 37, 13731–13736. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Abdul Wahid, M.; Jamil, M.M.; Azli, A.A.M.; Misbah, M.F. A review on biomass-based hydrogen production for renewable energy supply. Int. J. Energy Res. 2015, 39, 1597–1615. [Google Scholar] [CrossRef]
- Abd El-Hafiz, D.R.; Ebiad, M.A.; Elsalamony, R.A.; Mohamed, L.S. Highly stable nano Ce–La catalyst for hydrogen production from bio-ethanol. RSC Adv. 2015, 5, 4292–4303. [Google Scholar] [CrossRef]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Steam reforming of acetic acid using Ni/Al2O3 catalyst: Influence of crystalline phase of Al2O3 support. Int. J. Hydrogen Energy 2017, 42, 20729–20738. [Google Scholar] [CrossRef]
- Olsson, L.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme. Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current status of hydrogen production techniques by steam reforming of ethanol: A review. Energy Fuel 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Nguyen, A.-N.T.; Vu, K.B.; Giang, H.N.; Nguyen, T.-A.; Huynh, T.C.; Pham, H.D. Effect of supports and promoters on the performance of Ni-based catalysts in ethanol steam reforming. Chem. Eng. Technol. 2020, 43, 672–688. [Google Scholar] [CrossRef]
- Comas, J.; Mariño, F.; Laborde, M.; Amadeo, N. Bio-ethanol steam reforming on Ni/Al2O3 catalyst. Chem. Eng. J. 2004, 98, 61–68. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Insight into steam reforming of ethanol to produce hydrogen for fuel cells. Chem. Eng. J. 2006, 117, 39–49. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Contreras, J.L.; Salmones, J.; Colín-Luna, J.A.; Nuño, L.; Quintana, B.; Córdova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 production using the ethanol steam reforming (a review). Int. J. Hydrogen Energy 2014, 39, 18835–18853. [Google Scholar] [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward understanding metal-catalyzed ethanol reforming. ACS Catal. 2015, 5, 3841–3863. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/MxOy-Al2O3 (M=Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrogen Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Elias, K.F.M.; Lucrédio, A.F.; Assaf, E.M. Effect of CaO addition on acid properties of Ni–Ca/Al2O3 catalysts applied to ethanol steam reforming. Int. J. Hydrogen Energy 2013, 38, 4407–4417. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Gao, Y.; Qiao, Y.; Zheng, Q.; Guo, Z.; Zhao, Y.; O’Hare, D.; Wang, Q. Synthesis of LiAl2-layered double hydroxides for CO2 capture over a wide temperature range. J. Mater. Chem. A 2014, 2, 18454–18462. [Google Scholar] [CrossRef]
- Sikander, U.; Sufian, S.; Salam, M.A. A review of hydrotalcite based catalysts for hydrogen production systems. Int. J. Hydrogen Energy 2017, 42, 19851–19868. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liu, S.-J.; Uan, J.-Y. Controllable luminescence of a Li–Al layered double hydroxide used as a sensor for reversible sensing of carbonate. J. Mater. Chem.C 2019, 7, 11191–11206. [Google Scholar] [CrossRef]
- Tichit, D.; Gérardin, C.; Durand, R.; Coq, B. Layered double hydroxides: Precursors for multifunctional catalysts. Top. Catal. 2006, 39, 89–96. [Google Scholar] [CrossRef]
- Feng, J.-T.; Lin, Y.-J.; Evans, D.G.; Duan, X.; Li, D.-Q. Enhanced metal dispersion and hydrodechlorination properties of a Ni/Al2O3 catalyst derived from layered double hydroxides. J. Catal. 2009, 266, 351–358. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, S.; Wang, S.; Ma, X. Hydrogen production from ethanol steam reforming over nickel based catalyst derived from Ni/Mg/Al hydrotalcite-like compounds. Int. J. Hydrogen Energy 2010, 35, 6699–6708. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Lindo, M.; Carrero, A.; Calles, J.A. Hydrogen production by steam reforming of ethanol using Ni catalysts based on ternary mixed oxides prepared by coprecipitation. Int. J. Hydrog. Energy 2012, 37, 1985–1992. [Google Scholar] [CrossRef]
- Serna, C.J.; Rendon, J.L.; Iglesias, J.E. Crystal-chemical study of layered [Al2Li(OH)6]+ x-· nH2O. Clays. Clay Miner. 1982, 30, 180–184. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro’, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. Struct. Bond. 2006, 119, 1–87. [Google Scholar]
- Hernandez, M.J.; Ulibarri, M.A.; Cornejo, J.; Pena, M.J.; Serna, C.J. Thermal stability of aluminium hydroxycarbonates with monovalent cations. Thermochim. Acta 1985, 94, 257–266. [Google Scholar] [CrossRef]
- Shumaker, J.L.; Crofcheck, C.; Tackett, S.A.; Santillan-Jimenez, E.; Crocker, M. Biodiesel production from soybean oil using calcined Li–Al layered double hydroxide catalysts. Catal. Lett. 2007, 115, 56–61. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Structured Catalysts and Reactors; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Palma, V.; Ruocco, C.; Castaldo, F.; Ricca, A.; Boettge, D. Ethanol steam reforming over bimetallic coated ceramic foams: Effect of reactor configuration and catalytic support. Int. J. Hydrogen Energy 2015, 40, 12650–12662. [Google Scholar] [CrossRef]
- Santander, J.A.; Tonetto, G.M.; Pedernera, M.N.; López, E. Ni/CeO2–MgO catalysts supported on stainless steel plates for ethanol steam reforming. Int. J. Hydrogen Energy 2017, 42, 9482–9492. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A. Structured catalysts and reactors—Perspectives for demanding applications. Catal. Today 2020, 383, 5–14. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Spadaro, L.; Di Blasi, O.; Bonura, G.; Cavallaro, S. Steam reforming of bio-ethanol on alkali-doped Ni/MgO catalysts: Hydrogen production for mc fuel cell. Appl. Catal. A Gen. 2004, 270, 1–7. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; de Guibert, A. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Kamath, P.V.; Subbanna, G.N. Electroless nickel hydroxide: Synthesis and characterization. J. Appl. Electrochem. 1992, 22, 478–482. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, X.-G.; Fu, S.-Y. Preparation of urchinlike NiO nanostructures and their electrochemical capacitive behaviors. Mater. Res. Bull. 2006, 41, 620–627. [Google Scholar] [CrossRef]
- Duan, G.; Cai, W.; Luo, Y.; Sun, F. A hierarchically structured Ni(OH)2 monolayer hollow-sphere array and its tunable optical properties over a large region. Adv. Func. Mater. 2007, 17, 644–650. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Lee, D.H. Preparation of nano-NiO particles and evaluation of their catalytic activity in pyrolyzing biomass components. Energ. Fuel. 2008, 22, 16–23. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, N.; Liu, H.; He, Z. Microwave-assisted hydrothermal synthesis of Ni(OH)2 architectures and their in situ thermal convention to NiO. Adv. Powder Technol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Song, Q.S.; Li, Y.Y.; Chan, S.L.I. Physical and electrochemical characteristics of nanostructured nickel hydroxide powder. J. Appl. Electrochem. 2005, 35, 157–162. [Google Scholar] [CrossRef]
- Shangguan, E.; Chang, Z.; Tang, H.; Yuan, X.-Z.; Wang, H. Synthesis and characterization of high-density non-spherical Ni(OH)2 cathode material for Ni–MH batteries. Int. J. Hydrogen Energy 2010, 35, 9716–9724. [Google Scholar] [CrossRef]
- Zieliński, J. The effect of water on the reduction of nickel/alumina catalysts. Catal. Lett. 1992, 12, 389–394. [Google Scholar] [CrossRef]
- Zieliński, J. Effect of alumina on the reduction of surface nickel oxide; morphology of the surfaces of the surfaces of Ni/Al2O3 catalysts. J. Mol. Catal. 1993, 83, 197–206. [Google Scholar] [CrossRef]
- Zieliński, J. Effect of water on the reduction of nickel-alumina catalysts catalyst characterization by temperature-programmed reduction. J. Chem. Soc. Faraday Trans. 1997, 93, 3577–3580. [Google Scholar] [CrossRef]
- Shalvoy, R.B.; Davis, B.H.; Reucroft, P.J. Studies of the metal–support interaction in coprecipitated nickel on alumina methanation catalysts using x-ray photoelectron spectroscopy (xps). Surf. Interface Anal. 1980, 2, 11–16. [Google Scholar] [CrossRef]
- Jr, L.S.; Makovsky, L.E.; Stencel, J.M.; Brown, F.R.; Hercules, D.M. Surface spectroscopic study of tungsten-alumina catalysts using x-ray photoelectron, ion scattering, and raman spectroscopies. J. Phys. Chem. 1981, 85, 3700–3707. [Google Scholar]
- Meng, F.; Li, X.; Li, M.; Cui, X.; Li, Z. Catalytic performance of CO methanation over La-promoted Ni/Al2O3 catalyst in a slurry-bed reactor. Chem. Eng. J. 2017, 313, 1548–1555. [Google Scholar] [CrossRef]
- Czekaj, I.; Loviat, F.; Raimondi, F.; Wambach, J.; Biollaz, S.; Wokaun, A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS). Appl. Catal. A Gen. 2007, 329, 68–78. [Google Scholar] [CrossRef]
- Baker, R.T.K. Catalytic growth of carbon filaments. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Helveg, S.; Lo´ pez-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofiber growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef]
- Jeong, N.; Lee, J. Growth of filamentous carbon by decomposition of ethanol on nickel foam: Influence of synthesis conditions and catalytic nanoparticles on growth yield and mechanism. J. Catal. 2008, 260, 217–226. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Takanabe, K.; Basset, J.-M. Effect of NiAl2O4 formation on Ni/Al2O3 stability during dry reforming of methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Barbier, J. Deactivation of reforming catalysts by coking-a review. Appl. Catal. 1986, 23, 225–243. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Deactivation and coke accumulation during CO2/CH4 reforming over pt catalysts. J. Catal. 1999, 183, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.F.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Martins, A.A.; Mata, T.M.; Caetano, N.S.; Loureiro, J.M. Syngas production by bi-reforming methane on an Ni–K-promoted catalyst using hydrotalcites and filamentous carbon as a support material. RSC Adv. 2020, 10, 21158–21173. [Google Scholar] [CrossRef]
- Berteau, P.; Delmon, B. Modified aluminas: Relationship between activity in 1-butanol dehydration and acidity measured by NH3 TPD. Catal. Today 1989, 5, 121–137. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sanchez-Sanchez, M.C.; Fierro, J.L.G. Structure and activity of Pt-Ni catalysts supported on modified Al2O3 for ethanol steam reforming. J. Nanosci. Nanotechnol. 2015, 15, 6592–6603. [Google Scholar] [CrossRef]
- Yergaziyeva, G.Y.; Dossumov, K.; Mambetova, M.M.; Strizhak, P.Y.; Kurokawa, H.; Baizhomartov, B. Effect of Ni, La, and Ce oxides on a Cu/Al2O3 catalyst with low copper loading for ethanol non-oxidative dehydrogenation. Chem. Eng. Technol. 2021, 44, 1890–1899. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, B.S.; Wang, W.S.; Wang, F.; Zhang, Z.F. Mesoporous and macroporous alumina-supported nickel adsorbents for adsorptive desulphurization of commercial diesel. Adsorp. Sci. Technol. 2015, 33, 337–353. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, R.; Wang, Y.; Yang, M.; Guo, Y.; Wang, M.; Zhang, J.; Xu, J.; Xu, X.; Wang, X. Plasma assisted preparation of highly active NiAl2O4 catalysts for propane steam reforming. Int. J. Hydrogen Energy 2021, 46, 24931–24941. [Google Scholar] [CrossRef]
- Nivangune, N.; Kelkar, A. Selective synthesis of dimethyl carbonate via transesterification of propylene carbonate with methanol catalyzed by bifunctional Li-Al nano-composite. ChemistrySelect 2019, 4, 8574–8583. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Diez, V.K.; Xu, M.; Iglesia, E.; Apesteguia, C.R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Coleman, L.J.I.; Epling, W.; Hudgins, R.R.; Croiset, E. Ni/Mg–Al mixed oxide catalyst for the steam reforming of ethanol. Appl. Catal. A Gen. 2009, 363, 52–63. [Google Scholar] [CrossRef]
- Pavel, O.D.; Tichit, D.; Marcu, I.-C. Acido-basic and catalytic properties of transition-metal containing Mg–Al hydrotalcites and their corresponding mixed oxides. Appl. Clay Sci. 2012, 61, 52–58. [Google Scholar] [CrossRef]
- Antoniak-Jurak, K.; Kowalik, P.; Michalska, K.; Próchniak, W.; Bicki, R. Zn-Al mixed oxides decorated with potassium as catalysts for HT-WGS: Preparation and properties. Catalysts 2020, 10, 1094. [Google Scholar] [CrossRef]
- Marino, F.J.; Cerrella, E.G.; Duhalde, S.; Jobbagy, M.; Laborde, M.A. Hydrogen from steam reforming of ethanol. Characterization and performance of copper-nickel supported catalysts. Int. J. Hydrog. Energy 1998, 23, 1095–1101. [Google Scholar] [CrossRef]
- Marino, F.; Boveri, M.; Baronetti, G.; Laborde, M. Hydrogen production from steam reforming of bioethanol using Cu/Ni/K/γ-Al2O3 catalysts. Effect of Ni. Int. J. Hydrogen Energy 2001, 26, 665–668. [Google Scholar] [CrossRef]













| Catalyst | Conversion (%), Selectivity (mol%) and Deposition of Carbon Species (g/(gcat·h)) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XEtOH | H2 | CO2 | CO | CH4 | C2H4 | (C2H5)2O | CH3CHO | CH3COCH3 | C | |
| NaOH_T + R | 30.35 | 37.97 | 13.27 | 11.59 | 8.82 | 20.93 | 7.40 | 40.58 | 1.14 | 0.943 |
| NaHCO3_T + R | 21.94 | 74.04 | 30.84 | 13.91 | 5.80 | 11.01 | 2.57 | 43.21 | 0.77 | 0.869 |
| Element | NaOH_T + R (at.%) | NaHCO3_T + R (at.%) |
|---|---|---|
| Ni | 7.83 | 17.54 |
| Al | 10.16 | 13.73 |
| O | 82.01 | 68.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Chen, Y.-J.; Huang, W.-F.; Uan, J.-Y. Electrodeposition of a Li-Al Layered Double Hydroxide (LDH) on a Ball-like Aluminum Lathe Waste Strips in Structured Catalytic Applications: Preparation and Characterization of Ni-Based LDH Catalysts for Hydrogen Evolution. Catalysts 2022, 12, 520. https://doi.org/10.3390/catal12050520
Huang S-H, Chen Y-J, Huang W-F, Uan J-Y. Electrodeposition of a Li-Al Layered Double Hydroxide (LDH) on a Ball-like Aluminum Lathe Waste Strips in Structured Catalytic Applications: Preparation and Characterization of Ni-Based LDH Catalysts for Hydrogen Evolution. Catalysts. 2022; 12(5):520. https://doi.org/10.3390/catal12050520
Chicago/Turabian StyleHuang, Song-Hui, Yu-Jia Chen, Wen-Fu Huang, and Jun-Yen Uan. 2022. "Electrodeposition of a Li-Al Layered Double Hydroxide (LDH) on a Ball-like Aluminum Lathe Waste Strips in Structured Catalytic Applications: Preparation and Characterization of Ni-Based LDH Catalysts for Hydrogen Evolution" Catalysts 12, no. 5: 520. https://doi.org/10.3390/catal12050520