Heterojunction Design between WSe2 Nanosheets and TiO2 for Efficient Photocatalytic Hydrogen Generation
Abstract
:1. Introduction
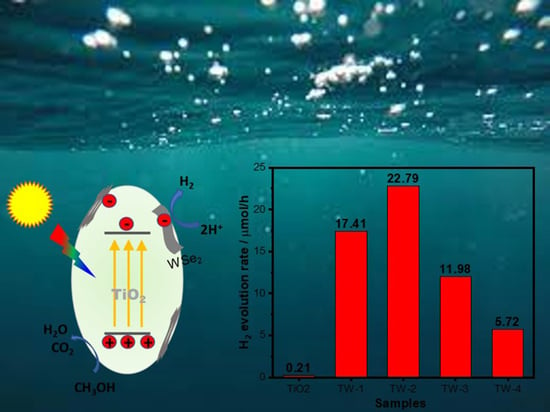
2. Results and Discussions
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.3. Sample Characterization
3.4. Photocatalytic Hydrogen Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Khan, M.M.; Rahman, A. Chalcogenides and chalcogenide-based heterostructures as photocatalysts for water splitting. Catalysts 2022, 12, 1338. [Google Scholar] [CrossRef]
- Serra, J.M.; Borrás-Morell, J.F.; García-Baños, B.; Balaguer, M.; Plaza-González, P.; Santos-Blasco, J.; Catalán-Martínez, D.; Navarrete, L.; Catalá-Civera, J.M. Hydrogen production via microwave-induced water splitting at low temperature. Nat. Energy 2020, 5, 910–919. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Ming, M.; Yuan, H.; Yang, S.; Wei, Z.; Lei, Q.; Lei, J.; Han, Z. Efficient red-light-driven hydrogen evolution with an anthraquinone organic dye. J. Am. Chem. Soc. 2022, 144, 19680–19684. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Yan, J.; Liu, S. Heteroepitaxial growth of core-shell ZnO/CdS heterostructure for efficient and stable photocatalytic hydrogen generation. Int. J. Hydrog. Energy 2022, 47, 34410–34420. [Google Scholar] [CrossRef]
- Willkomm, J.; Orchard, K.L.; Reynal, A.; Pastor, E.; Durrant, J.R.; Reisner, E. Dye-sensitized semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 2016, 45, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zong, S.; Cheng, C.; Shi, J.; Guo, P.; Guan, X.; Luo, B.; Shen, S.; Guo, L. Rapid high-temperature treatment on graphitic carbon nitride for excellent photocatalytic H2-evolution performance. Appl. Catal. B Environ. 2018, 233, 80–87. [Google Scholar] [CrossRef]
- Wang, M.; Xu, S.; Zhou, Z.; Dong, C.; Guo, X.; Chen, J.; Huang, Y.; Shen, S.; Chen, Y.; Guo, L.; et al. Atomically dispersed janus nickel sites on red phosphorus for photocatalytic overall water splitting. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204711. [Google Scholar]
- Guo, X.; Liu, X.; Yan, J.; Liu, S. Heterointerface engineering of ZnO/CdS heterostructures via ZnS layer for photocatalytic water splitting. Chem. A Eur. J. 2022, 144, e202202662. [Google Scholar]
- Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single molecule photocatalysis on TiO2 surfaces. Chem. Rev. 2019, 119, 11020–11041. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhu, Y.-N.; An, X.; Liu, L.; Cao, X.; Liu, H.; Qu, J. Defect modulation of Z-scheme TiO2/Cu2O photocatalysts for durable water splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Thanh Thuy, C.T.; Shin, G.; Jieun, L.; Kim, H.D.; Koyyada, G.; Kim, J.H. Self-doped carbon dots decorated TiO2 nanorods: A novel synthesis route for enhanced photoelectrochemical water splitting. Catalysts 2022, 12, 1281. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, X.; Cui, Y.; Fan, X.; Li, Z.; Ba, K.; Jia, G.; Zhang, H.; Zhang, H. Favorable energy band alignment of TiO2 anatase/rutile heterophase homojunctions yields photocatalytic hydrogen evolution with quantum efficiency exceeding 45.6%. Adv. Energy Mater. 2022, 12, 2200298. [Google Scholar] [CrossRef]
- Cheng, Q.; Yuan, Y.-J.; Tang, R.; Liu, Q.-Y.; Bao, L.; Wang, P.; Zhong, J.; Zhao, Z.; Yu, Z.-T.; Zou, Z. Rapid hydroxyl radical generation on (001)-facet-exposed ultrathin anatase TiO2 nanosheets for enhanced photocatalytic lignocellulose-to-H2 conversion. ACS Catal. 2022, 12, 2118–2125. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467. [Google Scholar] [CrossRef]
- AlSalka, Y.; Al-Madanat, O.; Curti, M.; Hakki, A.; Bahnemann, D.W. Photocatalytic H2 evolution from oxalic acid: Effect of cocatalysts and carbon dioxide radical anion on the surface charge transfer mechanisms. ACS Appl. Energy Mater. 2020, 3, 6678–6691. [Google Scholar] [CrossRef]
- Hakki, A.; AlSalka, Y.; Mendive, C.B.; Ubogui, J.; dos Santos Claro, P.C.; Bahnemann, D. Hydrogen production by heterogeneous photocatalysis. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 413–419. [Google Scholar]
- Wang, Y.; Liu, X.; Zheng, C.; Li, Y.; Jia, S.; Li, Z.; Zhao, Y. Tailoring TiO2 nanotube-interlaced graphite carbon nitride nanosheets for improving visible-light-driven photocatalytic performance. Adv. Sci. 2018, 5, 1700844. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liu, H.; Ning, S.; Ye, J. Localized surface plasmon resonance effect enhanced Cu/TiO2 core–shell catalyst for boosting CO2 hydrogenation reaction. Catal. Sci. Technol. 2022, 12, 6155–6162. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ding, P.; Wang, T.; Liu, L.-M.; An, X.; Yu, X. Facet-regulating local coordination of dual-atom cocatalyzed TiO2 for photocatalytic water splitting. ACS Catal. 2021, 11, 14669–14676. [Google Scholar] [CrossRef]
- Chen, Y.; Soler, L.; Armengol-Profitós, M.; Xie, C.; Crespo, D.; Llorca, J. Enhanced photoproduction of hydrogen on Pd/TiO2 prepared by mechanochemistry. Appl. Catal. B Environ. 2022, 309, 121275. [Google Scholar] [CrossRef]
- Sordello, F.; Pellegrino, F.; Prozzi, M.; Minero, C.; Maurino, V. Controlled periodic illumination enhances hydrogen production by over 50% on Pt/TiO2. ACS Catal. 2021, 11, 6484–6488. [Google Scholar] [CrossRef]
- Moon, H.S.; Hsiao, K.C.; Wu, M.C.; Yun, Y.; Hsu, Y.J.; Yong, K. Spatial separation of cocatalysts on Z-scheme organic/inorganic heterostructure hollow spheres for enhanced photocatalytic H2 evolution and in-depth analysis of the charge-transfer mechanism. Adv. Mater. 2022, 34, e2200172. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Y.; Liu, Y.; Yang, J.; Fujita, T.; Zeng, D. Fast one-pot synthesis of a Se-rich MnCdSe solid solution for highly efficient cocatalyst-free photocatalytic H2 evolution. Chem. Commun. 2022, 58, 6425–6428. [Google Scholar] [CrossRef]
- Cai, M.; Cao, S.; Zhou, Z.; Wang, X.; Shi, K.; Cheng, Q.; Xue, Z.; Du, X.; Shen, C.; Liu, X.; et al. Fabrication of Ni2P cocatalyzed CdS nanorods with a well-defined heterointerface for enhanced photocatalytic H2 evolution. Catalysts 2022, 12, 417. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.; Zhang, F.; Zhang, G.; Fan, X. Roles of two-dimensional transition metal dichalcogenides as cocatalysts in photocatalytic hydrogen evolution and environmental remediation. Ind. Eng. Chem. Res. 2017, 56, 4611–4626. [Google Scholar] [CrossRef]
- Eftekhari, A. Tungsten dichalcogenides (WS2, WSe2, and WTe2): Materials chemistry and applications. J. Mater. Chem. A 2017, 5, 18299–18325. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.J.; Lu, Q.; Zhao, M.; Yin, P.F.; Ma, Q.; Nam, G.H.; Li, B.; Chen, B.; Zhang, H. Preparation of CdSySe1-y-MoS2 heterostructures via cation exchange of pre-epitaxially synthesized Cu2−xSy-Se1−y-MoS2 for photocatalytic hydrogen evolution. Small 2021, 17, e2006135. [Google Scholar] [CrossRef]
- Jun, S.E.; Hong, S.; Choi, S.; Kim, C.; Ji, S.G.; Lee, S.A.; Yang, J.E.; Lee, T.H.; Sohn, W.; Kim, J.Y.; et al. Boosting unassisted alkaline solar water splitting using silicon photocathode with TiO2 nanorods decorated by edge-rich MoS2 nanoplates. Small 2021, 17, e2103457. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, Y.; Kang, S.; Guo, X.; Ma, Y.; Xing, M.; Zhu, Y.; Chai, Y.; Qiu, B. Trash to treasure: Photo reforming of plastic waste into commodity chemicals and hydrogen over MoS2-tipped CdS nanorods. ACS Catal. 2022, 12, 12823–12832. [Google Scholar] [CrossRef]
- Guo, X.; Guo, P.; Wang, C.; Chen, Y.; Guo, L. Few-layer WSe2 nanosheets as an efficient cocatalyst for improved photocatalytic hydrogen evolution over Zn0.1Cd0.9S nanorods. Chem. Eng. J. 2020, 383, 123183. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Liu, Y.; Jin, T.; Chen, Y.; Guo, L.; Lian, T. Enhanced light-driven charge separation and H2 generation efficiency in WSe2 nanosheet-semiconductor nanocrystal heterostructures. ACS Appl. Mater. Interfaces 2020, 12, 44769–44776. [Google Scholar] [CrossRef] [PubMed]
- Arun Joshi Reddy, K.; Amaranatha Reddy, D.; Hye Hong, D.; Gopannagari, M.; Putta Rangappa, A.; Praveen Kumar, D.; Kyu Kim, T. Impact of the number of surface-attached tungsten diselenide layers on cadmium sulfide nanorods on the charge transfer and photocatalytic hydrogen evolution rate. J. Colloid. Interface Sci. 2022, 608, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Kannichankandy, D.; Pataniya, P.M.; Sumesh, C.K.; Solanki, G.K.; Pathak, V.M. WSe2-PANI nanohybrid structure as efficient electrocatalyst for photo-enhanced hydrogen evolution reaction. J. Alloys Compd. 2021, 876, 160179. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Q.; Sun, Y.; Liu, K.; Cui, J.; Lu, C.; Dai, H. Fabrication of novel p-n-p heterojunctions ternary WSe2/In2S3/ZnIn2S4 to Enhance visible-light photocatalytic activity. J. Environ. Chem. Eng. 2022, 10, 108354. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Li, M.; Shen, H.; Zhang, W.; Zhang, J.; Liu, T.; Kong, X.; Bi, H. Metallic phase WSe2 nanoscrolls for the hydrogen evolution reaction. New J. Chem. 2022, 46, 8381–8384. [Google Scholar] [CrossRef]
- Qorbani, M.; Sabbah, A.; Lay, Y.; Kholimatussadiah, S.; Quadir, S.; Huang, C.; Shown, I.; Huang, Y.; Hayashi, M.; Chen, K.; et al. Atomistic insights into highly active reconstructed edges of monolayer 2H-WSe2 photocatalyst. Nat. Commun. 2022, 13, 1256. [Google Scholar] [CrossRef]
- Saruyama, M.; Pelicano, C.M.; Teranishi, T. Bridging electrocatalyst and cocatalyst studies for solar hydrogen production via water splitting. Chem. Sci. 2022, 13, 2824–2840. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Saruyama, M.; Takahata, R.; Sato, R.; Kitahama, Y.; Matsuzaki, H.; Yamada, T.; Hisatomi, T.; Domen, K.; Teranishi, T. Bimetallic synergy in ultrafine cocatalyst alloy nanoparticles for efficient photocatalytic water splitting. Adv. Funct. Mater. 2022, 32, 2202987. [Google Scholar] [CrossRef]
- Cantarella, M.; Gorrasi, G.; Mauro, A.; Scuderi, M.; Nicotra, G.; Fiorenza, R.; Scire, S.; Scalisi, M.; Crundo, M.; Privitera, V.; et al. Mechanical milling: A sustainable route to induce structural transformations in MoS2 for applications in the treatment of contaminated water. Sci. Rep. 2019, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Cho, M.H. Simple and large-scale construction of MoS2-g-C3N4 heterostructures using mechanochemistry for high performance electrochemical supercapacitor and visible light photocatalytic applications. Sci. Rep. 2017, 7, 43055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Jiang, S.; Huo, X.; Xia, R.; Muhire, E.; Gao, M. Facile preparation of a TiO2 quantum dot/graphitic carbon nitride heterojunction with highly efficient photocatalytic activity. Nanotechnology 2018, 29, 205702. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zong, S.; Tian, L.; Zhang, Y.; Shi, J. Construction of SrTiO3-LaCrO3 solid solutions with consecutive band structures for photocatalytic H2 evolution under visible light irradiation. Catalysts 2022, 12, 1123. [Google Scholar] [CrossRef]
- Tian, M.; Ma, M.; Xu, B.; Chen, C.; He, C.; Hao, Z.; Albilali, R. Catalytic removal of 1,2-dichloroethane over LaSrMnCoO6/H-ZSM-5 composite: Insights into synergistic effect and pollutant-destruction mechanism. Catal. Sci. Technol. 2018, 8, 4503–4514. [Google Scholar] [CrossRef]
- Tian, M.; Guo, X.; Dong, R.; Guo, Z.; Shi, J.; Yu, Y.; Cheng, M.; Albilali, R.; He, C. Insight into the boosted catalytic performance and chlorine resistance of nanosphere-like meso-macroporous CrOx/MnCo3Ox for 1,2-dichloroethane destruction. Appl. Catal. B Environ. 2019, 259, 118018. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.B.; Qin, Z.X.; Su, J.Z.; Guo, L.J. Facet-selective growth of cadmium sulfide nanorods on zinc oxide microrods: Intergrowth effect for improved photocatalytic performance. ChemCatChem 2018, 10, 153–158. [Google Scholar] [CrossRef]
- Biswas, M.; Ali, A.; Cho, K.Y.; Oh, W.C. Novel synthesis of WSe2-graphene-TiO2 ternary nanocomposite via ultrasonic technics for high photocatalytic reduction of CO2 into CH3OH. Ultrason. Sonochem. 2018, 42, 738–746. [Google Scholar] [CrossRef]
- Park, Y.J.; So, H.S.; Hwang, H.; Jeong, D.S.; Lee, H.J.; Lim, J.; Kim, C.G.; Shin, H.S. Synthesis of 1T WSe2 on an oxygen-containing substrate using a single precursor. ACS Nano 2022, 16, 11059–11065. [Google Scholar] [CrossRef]
- Smyth, C.M.; Addou, R.; McDonnell, S.; Hinkle, C.L.; Wallace, R.M. WSe2-contact metal interface chemistry and band alignment under high vacuum and ultra high vacuum deposition conditions. 2D Mater. 2017, 4, 025084. [Google Scholar] [CrossRef]
- Xia, M.; Ning, J.; Wang, D.; Feng, X.; Wang, B.; Guo, H.; Zhang, J.; Hao, Y. Ammonia-assisted synthesis of gypsophila-like 1T-WSe2/graphene with enhanced potassium storage for all-solid-state supercapacitor. Chem. Eng. J. 2021, 405, 126611. [Google Scholar] [CrossRef]
- Kadam, S.R.; Enyashin, A.N.; Houben, L.; Bar-Ziv, R.; Bar-Sadan, M. Ni-WSe2 nanostructures as efficient catalysts for electrochemical hydrogen evolution reaction (HER) in acidic and alkaline media. J. Mater. Chem. A 2020, 8, 1403–1416. [Google Scholar] [CrossRef]
- Zhang, K.; Fujitsuka, M.; Du, Y.; Majima, T. 2D/2D heterostructured CdS/WS2 with efficient charge separation improving H2 evolution under visible light irradiation. ACS Appl. Mater. Interfaces 2018, 10, 20458–20466. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, S.; Guo, X.; Xiao, Y.; Yin, Z.; Yang, N.; Bao, Y.; Zhu, X.; Jin, S.; Feng, Z. Water-stable nickel metal-organic framework nanobelts for cocatalyst-free photocatalytic water splitting to produce hydrogen. J. Am. Chem. Soc. 2022, 144, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.; Goswami, T.; Yadav, D.K.; Ghorai, N.; Shukla, A.; Kaur, G.; Kaur, A.; Ghosh, H.N. Ultrafast hot electron transfer and trap-state mediated charge carrier separation toward enhanced photocatalytic activity in g-C3N4/ZnIn2S4 heterostructure. J. Phys. Chem. Lett. 2021, 12, 11865–11872. [Google Scholar] [CrossRef]
- Feng, K.; Sun, T.; Hu, X.; Fan, J.; Yang, D.; Liu, E. 0D/2D Co0.85Se/TiS2 p–n heterojunction for enhanced photocatalytic H2 Evolution. Catal. Sci. Technol. 2022, 12, 4893–4902. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Miao, Y.; Zhou, C.; Zhang, L.P.; Wu, L.Z.; Zhang, T. Accelerating electron-transfer dynamics by TiO2 immobilized reversible single-atom copper for enhanced artificial photosynthesis of urea. Adv. Mater. 2022, e2207793. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.; Liu, L.; Palma, B.; Hu, Z.; Renneckar, S.; Larter, S.; Li, Y.; Kibria, M.G.; Hu, J.; et al. n-p heterojunction of TiO2-NiO core-shell structure for efficient hydrogen generation and lignin photoreforming. J. Colloid. Interface Sci. 2021, 585, 694–704. [Google Scholar] [CrossRef]
- Zhao, C.; Tao, W.; Chen, Z.; Zhou, H.; Zhang, C.; Lin, J.; Zhu, H. Ultrafast electron transfer with long-lived charge separation and spin polarization in WSe2/C60 heterojunction. J. Phys. Chem. Lett. 2021, 12, 3691–3697. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Qin, Z.; Wang, M.; Guo, L. One-step hydrothermal synthesis of ZnxCd1-XS/ZnO heterostructures for efficient photocatalytic hydrogen production. Int. J. Hydrog. Energy 2016, 41, 15208–15217. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, D.; Yu, H.; Fan, J.; Yu, J. Novel amorphous nicus H2-evolution cocatalyst: Optimizing surface hydrogen desorption for efficient photocatalytic activity. Chem. Eng. J. 2021, 419, 129652. [Google Scholar] [CrossRef]
- Xu, L.; Li, L.; Ao, Y.; Long, F.; Guan, J. Engineering highly efficient photocatalysts for hydrogen production by simply regulating the solubility of insoluble compound cocatalysts. Int. J. Hydrogen Energy 2014, 39, 11486–11493. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, P.; Zhu, W.; Li, G.; Zhang, D.; Li, H. Copper nanowires: A substitute for noble metals to enhance photocatalytic H2 generation. Nano Lett 2015, 15, 4853–4858. [Google Scholar] [CrossRef]
- Du, F.; Lu, H.; Lu, S.; Wang, J.; Xiao, Y.; Xue, W.; Cao, S. Photodeposition of amorphous MoSX cocatalyst on TiO2 nanosheets with {001} facets exposed for highly efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2018, 43, 3223–3234. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Chen, F.; Yu, H. Ni nanoparticles as electron-transfer mediators and NiSx as interfacial active sites for coordinative enhancement of H2-evolution performance of TiO2. Chinese J. Catal. 2019, 40, 343–351. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Liu, X.; Shan, J.; Zhao, G.; Liu, S. Heterojunction Design between WSe2 Nanosheets and TiO2 for Efficient Photocatalytic Hydrogen Generation. Catalysts 2022, 12, 1668. https://doi.org/10.3390/catal12121668
Guo X, Liu X, Shan J, Zhao G, Liu S. Heterojunction Design between WSe2 Nanosheets and TiO2 for Efficient Photocatalytic Hydrogen Generation. Catalysts. 2022; 12(12):1668. https://doi.org/10.3390/catal12121668
Chicago/Turabian StyleGuo, Xu, Xing Liu, Jing Shan, Guangtao Zhao, and Shengzhong (Frank) Liu. 2022. "Heterojunction Design between WSe2 Nanosheets and TiO2 for Efficient Photocatalytic Hydrogen Generation" Catalysts 12, no. 12: 1668. https://doi.org/10.3390/catal12121668