Selective Hydrogenation of 2-Methyl-3-butyn-2-ol in Microcapillary Reactor on Supported Intermetallic PdZn Catalyst, Effect of Support Doping on Stability and Kinetic Parameters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Composition Analysis of Catalysts and Coatings
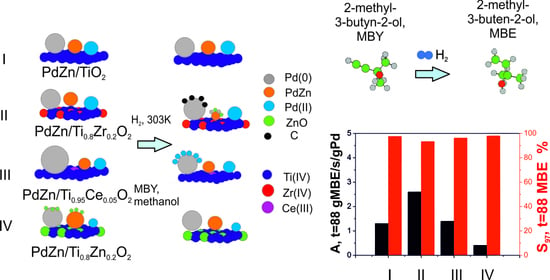
2.2. Hydrogenation of 2-Methyl-3-butyn-2-ol on PdZn/TixM1−xOy Coatings in a Microcapillary Reactor
2.3. Hydrogenation of 2-Methyl-3-butyn-2-ol on PdZn/TixM1−xOy Coatings after Different Pretreatments
3. Materials and Methods
3.1. Synthesis of PdZn/TixM1−xO2 (M = Zr, Ce, Zn) Coatings
3.2. Investigation of the Physicochemical Properties of TixM1−xO2 Composites (M = Zr, Ce, Zn) and PdZn/Tix Zr1−xO2 Catalysts and Coatings
3.3. Catalytic Tests
3.4. Calculations of Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renken, A.; Kiwi-Minsker, L. Microstructured Catalytic Reactors. Adv. Catal. 2010, 53, 47–122. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of Microreactor Technology and Flow Chemistry to the Development of Green and Sustainable Synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebrov, E.V.; Berenguer-Murcia, A.; Skelton, H.E.; Johnson, B.F.G.; Wheatley, A.E.H.; Schouten, J.C. Capillary Microreactors Wall-Coated with Mesoporous Titania Thin Film Catalyst Supports. Lab Chip 2009, 9, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Rebrov, E.V.; Klinger, E.A.; Berenguer-Murcia, A.; Sulman, E.M.; Schouten, J.C. Selective Hydrogenation of 2-Methyl-3-Butyne-2-Ol in a Wall-Coated Capillary Microreactor with a Pd25Zn75/TiO2 Catalyst. Org. Process Res. Dev. 2009, 13, 991–998. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Rebrov, E.V. Novel Synthesis of Thick Wall Coatings of Titania Supported Bi Poisoned Pd Catalysts and Application in Selective Hydrogenation of Acetylene Alcohols in Capillary Microreactors. Lab Chip 2015, 15, 1952–1960. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Ismagilov, Z.R. Capillary Microreactor with a Catalytic Coating Based on Mesoporous Titanium Dioxide for the Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol. Kinet. Catal. 2016, 57, 501–507. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Cherepanova, S.V.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Semi-Hydrogenation of 2-Methyl-3-Butyn-2-Ol on Pd-Zn Nanoalloys Prepared by Polyol Method: Effect of Composition and Heterogenization. Appl. Catal. A 2018, 549, 245–253. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Yu Stakheev, A. Formation of Supported Intermetallic Nanoparticles in the Pd–Zn/α-Al2O3 Catalyst. Kinet. Catal. 2017, 58, 499–507. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Rassolov, A.V.; Baeva, G.N.; Mashkovsky, I.S.; Murzin, V.Y.; Zubavichus, Y.V.; Stakheev, A.Y. Pd–Cu Catalyst Prepared from Heterobimetallic PdCu2(OAc)6: An XRD-EXAFS Study and Activity/Selectivity in the Liquid-Phase Hydrogenation of a C≡C Bond. Mendeleev Commun. 2016, 26, 502–504. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Rassolov, A.V.; Mashkovsky, I.S.; Baeva, G.N.; Tkachenko, O.P.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Performance of a Bimetallic Pd–In Catalyst in the Selective Liquid-Phase Hydrogenation of Internal and Terminal Alkynes. Mendeleev Commun. 2016, 26, 494–496. [Google Scholar] [CrossRef]
- Khan, N.A.; Shaikhutdinov, S.; Freund, H.J. Acetylene and Ethylene Hydrogenation on Alumina Supported Pd-Ag Model Catalysts. Catal. Letters 2006, 108, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.K.; Cherkasov, N.; Pérez-Barrado, E.; Aho, A.; Murzin, D.Y.; Ibhadon, A.O.; Francesconi, M.G. Pd 3 Sn Nanoparticles on TiO 2 and ZnO Supports as Catalysts for Semi-Hydrogenation: Synthesis and Catalytic Performance. Appl. Catal. A Gen. 2017, 544, 40–45. [Google Scholar] [CrossRef]
- Niu, W.; Gao, Y.; Zhang, W.; Yan, N.; Lu, X. Pd-Pb Alloy Nanocrystals with Tailored Composition for Semihydrogenation: Taking Advantage of Catalyst Poisoning. Angew. Chemie - Int. Ed. 2015, 54, 8271–8274. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Yarulin, A.; Jin, M.; Xia, Y.; Kiwi-Minsker, L. Structure Sensitivity of Alkynol Hydrogenation on Shape- and Size-Controlled Palladium Nanocrystals: Which Sites Are Most Active and Selective? J. Am. Chem. Soc. 2011, 133, 12787–12794. [Google Scholar] [CrossRef]
- Yarulin, A.E.; Crespo-Quesada, R.M.; Egorova, E.V.; Kiwi-Minsker, L.L. Structure Sensitivity of Selective Acetylene Hydrogenation over the Catalysts with Shape-Controlled Palladium Nanoparticles. Kinet. Catal. 2012, 53, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Protasova, L.N.; Rebrov, E.V.; Glazneva, T.S.; Berenguer-Murcia, A.; Ismagilov, Z.R.; Schouten, J.C. Control of the Thickness of Mesoporous Titania Films for Application in Multiphase Catalytic Microreactors. J. Catal. 2010, 271, 161–169. [Google Scholar] [CrossRef]
- Muraza, O.; Rebrov, E.V.; de Croon, M.H.J.M.; Schouten, J.C. Enhancement of the Stability of Microporous Silica Films in Non-Aqueous Solvents at Elevated Temperature. Microporous Mesoporous Mater. 2009, 124, 20–29. [Google Scholar] [CrossRef]
- Muraza, O.; Rebrov, E.V.; Berenguer-Murcia, A.; de Croon, M.H.J.M.; Schouten, J.C. Selectivity Control in Hydrogenation Reactions by Nanoconfinement of Polymetallic Nanoparticles in Mesoporous Thin Films. Appl. Catal. A Gen. 2009, 368, 87–96. [Google Scholar] [CrossRef]
- Protasova, L.N.; Rebrov, E.V.; Skelton, H.E.; Wheatley, A.E.H.; Schouten, J.C. A Kinetic Study of the Liquid-Phase Hydrogenation of Citral on Au/TiO2 and Pt–Sn/TiO2 Thin Films in Capillary Microreactors. Appl. Catal. A Gen. 2011, 399, 12–21. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Ismagilov, Z.R. Improved Thermal Stability of PdZn/TiO2coating by Ce Doping. Surf. Eng. 2015, 31, 78–83. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Ismagilov, Z.R. Development, Synthesis, and Study of Sanomaterials of Titania Doped by Zirconium for Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol in a Microcapillary Reactor. Kinet. Catal. 2019, 60, 474–483. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Li, S.; Feng, B.; Fu, Y.; Zhang, F.; Chen, D.L.; Zhu, W. PdZn Intermetallic Compound Stabilized on ZnO/Nitrogen-Decorated Carbon Hollow Spheres for Catalytic Semihydrogenation of Alkynols. Nano Res. 2022, 15, 3090–3098. [Google Scholar] [CrossRef]
- Vernuccio, S.; Goy, R.; Rudolf Von Rohr, P.; Medlock, J.; Bonrath, W. Hydrogenation of 2-Methyl-3-Butyn-2-Ol over a Pd/ZnO Catalyst: Kinetic Model and Selectivity Study. React. Chem. Eng. 2016, 1, 445–453. [Google Scholar] [CrossRef]
- Vernuccio, S.; Goy, R.; Meier, A.; Rudolf von Rohr, P.; Medlock, J. Kinetics and Mass Transfer of the Hydrogenation of 2-Methyl-3-Butyn-2-Ol in a Structured Pd/ZnO/Al2O3 Reactor. Chem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Wang, X.B.; Li, W.Y.; Liang, C. Intermetallic PdZn Nanoparticles Catalyze the Continuous-Flow Hydrogenation of Alkynols to Cis-Enols. Commun. Chem. 2021, 4, 175. [Google Scholar] [CrossRef]
- González-Fernández, A.; Pischetola, C.; Kiwi-Minsker, L.; Cárdenas-Lizana, F. Partial Hydrogenation of 2-Methyl-3-Butyn-2-Ol over Pd/ZnO: Effect of Reduction Temperature on Alloy Formation and Catalytic Response. J. Phys. Chem. C 2020, 124, 3681–3691. [Google Scholar] [CrossRef]
- Tew, M.W.; Emerich, H.; Van Bokhoven, J.A. Formation and Characterization of PdZn Alloy: A Very Selective Catalyst for Alkyne Semihydrogenation. J. Phys. Chem. C 2011, 115, 8457–8465. [Google Scholar] [CrossRef]
- Cherkasov, N.; Denissenko, P.; Deshmukh, S.; Rebrov, E.V. Gas-Liquid Hydrogenation in Continuous Flow – The Effect of Mass Transfer and Residence Time in Powder Packed-Bed and Catalyst-Coated Reactors. Chem. Eng. J. 2020, 379, 122292. [Google Scholar] [CrossRef]
- Asano, S.; Adams, S.J.; Tsuji, Y.; Yoshizawa, K.; Tahara, A.; Hayashi, J.I.; Cherkasov, N. Homogeneous Catalyst Modifier for Alkyne Semi-Hydrogenation: Systematic Screening in an Automated Flow Reactor and Computational Study on Mechanisms. React. Chem. Eng. 2022, 7, 1818–1826. [Google Scholar] [CrossRef]
- Cherkasov, N.; Bai, Y.; Rebrov, E. Process Intensification of Alkynol Semihydrogenation in a Tube Reactor Coated with a Pd/ZnO Catalyst. Catalyst. 2017, 7, 358. [Google Scholar] [CrossRef]
- Cherkasov, N.; Al-Rawashdeh, M.; Ibhadon, A.O.; Rebrov, E.V. Scale up Study of Capillary Microreactors in Solvent-Free Semihydrogenation of 2-Methyl-3-Butyn-2-Ol. Catal. Today 2016, 273, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Semagina, N.; Grasemann, M.; Xanthopoulos, N.; Renken, A.; Kiwi-Minsker, L. Structured Catalyst of Pd/ZnO on Sintered Metal Fibers for 2-Methyl-3-Butyn-2-Ol Selective Hydrogenation. J. Catal. 2007, 251, 213–222. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Ismagilov, Z.R. Coating the Internal Surface of a Capillary Microreactor for the Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol by PdxZn1 – x/TiO2 Catalysts. A Kinetic Study. Kinet. Catal. 2018, 59, 450–458. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Tuzikov, F.V.; Larichev, Y.V.; Ismagilov, Z.R. Palladium-Zinc Catalysts on Mesoporous Titania Prepared by Colloid Synthesis. II. Synthesis and Characterization of PdZn/TiO2 Coating on Inner Surface of Fused Silica Capillary. J. Nanoparticle Res. 2012, 14, 1088. [Google Scholar] [CrossRef]
- Carter, J.H.; Shah, P.M.; Nowicka, E.; Freakley, S.J.; Morgan, D.J.; Golunski, S.; Hutchings, G.J. Enhanced Activity and Stability of Gold/Ceria-Titania for the Low-Temperature Water-Gas Shift Reaction. Front. Chem. 2019, 7, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Ran, R.; Wu, X.; Si, Z.; Kang, F.; Weng, D. Progress on Metal-Support Interactions in Pd-Based Catalysts for Automobile Emission Control. J. Environ. Sci. (China) 2023, 125, 401–426. [Google Scholar] [CrossRef]
- Wessels, K.; Minnermann, M.; Rathousky, J.; Wark, M.; Oekermann, T. Influence of Calcination Temperature on the Photoelectrochemical and Photocatarytic Properties of Porous TiO2 Films Electrodeposited from Ti(IV)-Alkoxide Solution. J. Phys. Chem. C 2008, 112, 15122–15128. [Google Scholar] [CrossRef]
- Juliet, S.S.; Ramalingom, S.; Ravidhas, C.; Raj, A.M.E. Effect of Calcination Temperature on Titanium Oxide Nanocrystallites in the Anatase Phase Synthesized By Sol-Gel Route. IOSR J. Appl. Phys. 2017, 9, 32–39. [Google Scholar] [CrossRef]
- Nikoshvili, L.Z.; Makarova, A.S.; Lyubimova, N.A.; Bykov, A.V.; Sidorov, A.I.; Tyamina, I.Y.; Matveeva, V.G.; Sulman, E.M. Kinetic Study of Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol over Pd-Containing Hypercrosslinked Polystyrene. Catal. Today 2015, 256, 231–240. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Capillary Microreactor with PdZn/(Ti, Ce)O2 Coating for Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol. Chem. Eng. Process. - Process Intensif. 2021, 159, 108240. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Kerzhentsev, M.A.; Ismagilov, Z.R. Internal Surface Coating of a Capillary Microreactor for the Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol Using a PdZn/TiO2 Catalyst. The Effect of the Catalyst’s Activation Conditions on Its Catalytic Properties. Kinet. Catal. 2018, 59, 347–356. [Google Scholar] [CrossRef]
- Naganuma, T.; Traversa, E. Stability of the Ce 3+ Valence State in Cerium Oxide Nanoparticle Layers. Nanoscale 2012, 4, 4950–4953. [Google Scholar] [CrossRef] [PubMed]
- Rameshan, C.; Stadlmayr, W.; Weilach, C.; Penner, S.; Lorenz, H.; Hävecker, M.; Blume, R.; Rocha, T.; Teschner, D.; Knop-Gericke, A.; et al. Subsurface-Controlled CO2 Selectivity of PdZn near-Surface Alloys in H2 Generation by Methanol Steam Reforming. Angew. Chemie - Int. Ed. 2010, 49, 3224–3227. [Google Scholar] [CrossRef] [PubMed]
- Engels, V.; Jefferson, D.; Benaskar, F.; Thune, P.; Berenguer-Murcia, A.; Johnson, B.; Wheatley, A. Nanoparticulate PdZn - Pathways towards the Synthetic Control of Nanosurface Properties. Nanotechnology 2011, 22, 205701. [Google Scholar] [CrossRef]
- Holzapfel, H.H.; Wolfbeisser, A.; Rameshan, C.; Weilach, C.; Rupprechter, G. PdZn Surface Alloys as Models of Methanol Steam Reforming Catalysts: Molecular Studies by LEED, XPS, TPD and PM-IRAS. Top. Catal. 2014, 57, 1218–1228. [Google Scholar] [CrossRef]
- Doyle, A.M.; Shaikhutdinov, S.K.; Jackson, S.D.; Freund, H.J. Hydrogenation on Metal Surfaces: Why Are Nanoparticles More Active than Single Crystals? Angew. Chemie Int. Ed. 2003, 42, 5240–5243. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Andanson, J.M.; Yarulin, A.; Lim, B.; Xia, Y.; Kiwi-Minsker, L. UV-Ozone Cleaning of Supported Poly(Vinylpyrrolidone)-Stabilized Palladium Nanocubes: Effect of Stabilizer Removal on Morphology and Catalytic Behavior. Langmuir 2011, 27, 7909–7916. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Matus, E.V.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol Catalyzed by Embedded Polymer-Protected PdZn Nanoparticles. J. Nanoparticle Res. 2015, 17, 475. [Google Scholar] [CrossRef]
- Wowsnick, G.; Teschner, D.; Armbrüster, M.; Kasatkin, I.; Girgsdies, F.; Grin, Y.; Schlögl, R.; Behrens, M. Surface Dynamics of the Intermetallic Catalyst Pd2Ga, Part II - Reactivity and Stability in Liquid-Phase Hydrogenation of Phenylacetylene. J. Catal. 2014, 309, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Mao, S.; Li, J.; Li, M.; Chen, P.; Li, H.; Chen, Z.; Wang, Y. PdZn Intermetallic on a CN@ZnO Hybrid as an Efficient Catalyst for the Semihydrogenation of Alkynols. J. Catal. 2017, 350, 13–20. [Google Scholar] [CrossRef]
- Armbruster, M.; Behrens, M.; Cinquini, F.; Fottinger, K.; Grin, Y.; Haghofer, A.; Klotzer, B.; Knop-Gericke, A.; Lorenz, H.; Ota, A.; et al. How to Control the Selectivity of Palladium-Based Catalysts in Hydrogenation Reactions: The Role of Subsurface Chemistry. ChemCatChem 2012, 4, 1048–1063. [Google Scholar] [CrossRef]
- Jackson, S.D.; Shaw, L.A. The Liquid-Phase Hydrogenation of Phenyl Acetylene and Styrene on a Salladium /Carbon Catalyst. Appl. Catal. A, Gen. 1996, 134, 91–99. [Google Scholar] [CrossRef]
- Jackson, S.D.; Casey, N.J.; Huntington, I.J.; Ts, C. Characterization of the Carboneceous Deposit Formed during the Selective Hydrogenation of Propyne over Palladium/Zirconia. Stud. Surf. Sci. Catal. 1994, 88, 313–318. [Google Scholar] [CrossRef]
- Dominguez-Dominguez, S.; Berenguer-Murcia, A.; Cazorla-Amoros, D.; Linares-Solano, A. Semihydrogenation of Phenylacetylene Catalyzed by Metallic Nanoparticles Containing Noble Metals. J. Catal. 2006, 243, 74–81. [Google Scholar] [CrossRef]
- AGILENT. Available online: https://www.agilent.com/search/?Ntt=160-2530-10 (accessed on 15 January 2020).
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 EV. J. Electron Spectros. Relat. Phenomena 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Semagina, N.; Joannet, E.; Parra, S.; Sulman, E.; Renken, A.; Kiwi-Minsker, L. Palladium Nanoparticles Stabilized in Block-Copolymer Micelles for Highly Selective 2-Butyne-1,4-Diol Partial Hydrogenation. Appl. Catal. A Gen. 2005, 280, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Kashid, M.N.; Renken, A.; Kiwi-Minsker, L. (Eds.) Microstructured Devices for Chemical Processing; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; ISBN 9783527685226. [Google Scholar]
- Duca, D.; Liotta, L.F.; Deganello, G. Selective Hydrogenation of Phenylacetylene on Pumice-Supported Palladium Catalysts. J. Catal. 1995, 154, 69–79. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; McCue, A.J.; Anderson, J.A.; Johnston, S.K. Palladium-Bismuth Intermetallic and Surface-Poisoned Catalysts for the Semi-Hydrogenation of 2-Methyl-3-Butyn-2-Ol. Appl. Catal. A Gen. 2015, 497, 22–30. [Google Scholar] [CrossRef]
- Singh, U.K.; Albert Vannice, M. Liquid-Phase Hydrogenation of Citral over Pt/SiO2 Catalysts: I. Temperature Effects on Activity and Selectivity. J. Catal. 2000, 191, 165–180. [Google Scholar] [CrossRef]









| Supports | Calcination Temperature, K | Specific Surface Area, m2/g | Pore Size, nm | Pore Volume, cm3/g | Crystallite Size, nm |
|---|---|---|---|---|---|
| TiO2 | 673 | 151.8 | 3.8 | 0.132 | 6 |
| TiO2 | 873 | 23.4 | 10.2 | 0.061 | 22 |
| Ti0.95Ce0.05O2 | 673 | 261.4 | 4.0 | 0.22 | amorphous |
| Ti0.95Ce0.05O2 | 873 | 57.5 | 8.9 | 0.144 | 9 |
| Ti0.80Zr0.20O2 Ti0.80Zr0.20O2 | 673 | 185.5 | 3.6 | 0.084 | 9 |
| 873 | 128.6 | 5.9 | 0.236 | 15 | |
| Ti0.80Zn0.20O1.8 Ti0.80Zn0.20O1.8 | 673 | 250.9 | 4.2 | 0.253 | amorphous |
| 873 | 51 | 13.5 | 0.217 | 25 |
| Sample | MBY Concentration, t=28, mol/L | Q, t = 28, gMBE/day | A, t = 28, gMBE/s/gPd | S97, t = 28 % | MBY Concentration, t = 88, mol/L | Q, t = 88, gMBE/day | A, t = 88, gMBE/s/gPd | S97, t = 88 % |
|---|---|---|---|---|---|---|---|---|
| PdZn/TiO2 | 0.8 | 3.6 | 1.5 | 96.7 | 2.2 | 4.1 | 1.3 | 97.6 |
| PdZn/Ti0.8Zr0.2O2 | 1.0 | 6.3 | 1.4 | 96.8 | 2.0 | 12.0 | 2.6 | 93.4 |
| PdZn/Ti0.95Ce0.05O2 | 1.0 | 3.6 | 1.8 | 93.7 | 2.0 | 2.9 | 1.4 | 96.3 |
| PdZn/Ti0.8Zn0.2O2 | 0.4 | 0.43 | 0.12 | 97.6 | 1.0 | 1.56 | 0.41 | 98.0 |
| Parameter | PdZn/TiO2 | PdZn/Ti0.8Zr0.2O2 | PdZn/Ti0.95Ce0.05O2.08 | PdZn/Ti0.8Zn0.2O1.8 |
|---|---|---|---|---|
| k1’/mol/L/s/gPd | 714 | 788 | 1258 | 186 |
| k2’mol/L/s/gPd | 132 | 176 | 941 | 14 |
| k3’/mol/L/s/gPd | 20 | 41 | 0.0001 | 5 |
| KMBY/L/mol | 61 | 43 | 30 | 56 |
| KMBE/L/mol | 0.8 | 0.6 | 1 | 0.3 |
| KMBA/L/mol | 8 | 0.01 | 0.001 | 1 |
| KMBE/KMBY | 0.013 | 0.014 | 0.031 | 0.005 |
| KMBA/KMBE | 10 | 0.017 | 0.001 | 3.3 |
| KMBA/KMBY | 0.13 | 2.3 × 10−4 | 3.3 × 10−5 | 0.02 |
| σMBY, % | 25.0 | 7.5 | 24.0 | 3.3 |
| σMBE, % | 5.3 | 3.7 | 3.9 | 0.8 |
| σMBA, % | 13.0 | 8.0 | 11.6 | 20.7 |
| Q, gMBE/day | 3.6 | 6.3 | 3.6 | 0.42 |
| S97,% | 96.7 | 96.8 | 93.7 | 97.5 |
| Catalyst | Pretreatment | Pd3d5/2 | Zn2p3/2 | Ce3d5/2 | Pd/Ti | Pd/Zn | At. Conc. C, % | At. Conc. Zn,% |
|---|---|---|---|---|---|---|---|---|
| PdZn/TiO2 | H2/Ar, 573 K, 2 h | 335.1 | 1022 | - | 0.005 | 0.17 | 52.4 | 0.31 |
| PdZn/TiO2 | Air, 573 K, 2 h, H2/Ar, 573 K, 2 h | 335.3 | 1022.3 | - | 0.006 | 0.21 | 26.1 | 0.53 |
| PdZn/Ti0.95Ce0.05O2 | H2/Ar, 573 K, 2 h | 335.1 | 1022 | 882.3 | 0.006 | 2.0 | 50.9 | 0.04 |
| PdZn/Ti0.95Ce0.05O2 | Air, 573 K, 2 h, H2/Ar, 573 K, 2 h | 335.3 | 1022.2 | 882.2 | 0.009 | 2.3 | 32.1 | 0.07 |
| PdZn/Ti0.8Zn0.2O1.8 | H2/Ar, 573 K, 2 h | 335.1 | 1022.3 | - | 0.003 | 0.005 | 43.3 | 5.84 |
| PdZn/Ti0.8Zn0.2O1.8 | Air, 573 K, 2 h, H2/Ar, 573 K, 2 h | 335.2 | 1022.3 | - | 0.004 | 0.007 | 26.3 | 7.2 |
| Catalyst | Pretreatment | Pd(0) (%) | PdZn (%) | Pd(II) (%) |
|---|---|---|---|---|
| PdZn/TiO2 | H2/Ar, 573 K, 2 h | 48.9 | 23.6 | 27.5 |
| PdZn/TiO2 | Air, 573 K, 2 h, H2/Ar, 573 K, 2 h | 50.5 | 27.6 | 21.9 |
| PdZn/Ti0.95Ce0.05O2 | H2/Ar, 573 K, 2 h | 60.3 | 19.5 | 20.2 |
| PdZn/Ti0.95Ce0.05O2 | Air, 573 K, 2 h, H2/Ar, 573 K, 2 h | 46.1 | 18.1 | 35.8 |
| PdZn/Ti0.8Zn0.2O1.8 | H2/Ar, 573 K, 2 h | 52.6 | 36.0 | 11.4 |
| PdZn/Ti0.8Zn0.2O1.8 | Air, 573 K, 2 h, H2/Ar, 573 K, 2 h | 58.1 | 31.8 | 10.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okhlopkova, L.; Prosvirin, I.; Kerzhentsev, M.; Ismagilov, Z. Selective Hydrogenation of 2-Methyl-3-butyn-2-ol in Microcapillary Reactor on Supported Intermetallic PdZn Catalyst, Effect of Support Doping on Stability and Kinetic Parameters. Catalysts 2022, 12, 1660. https://doi.org/10.3390/catal12121660
Okhlopkova L, Prosvirin I, Kerzhentsev M, Ismagilov Z. Selective Hydrogenation of 2-Methyl-3-butyn-2-ol in Microcapillary Reactor on Supported Intermetallic PdZn Catalyst, Effect of Support Doping on Stability and Kinetic Parameters. Catalysts. 2022; 12(12):1660. https://doi.org/10.3390/catal12121660
Chicago/Turabian StyleOkhlopkova, Lyudmila, Igor Prosvirin, Mikhail Kerzhentsev, and Zinfer Ismagilov. 2022. "Selective Hydrogenation of 2-Methyl-3-butyn-2-ol in Microcapillary Reactor on Supported Intermetallic PdZn Catalyst, Effect of Support Doping on Stability and Kinetic Parameters" Catalysts 12, no. 12: 1660. https://doi.org/10.3390/catal12121660