Hydrodeoxygenation–Isomerization of Methyl Palmitate over SAPO-11-Supported Ni-Phosphide Catalysts
Abstract
:1. Introduction
2. Results and Discussion
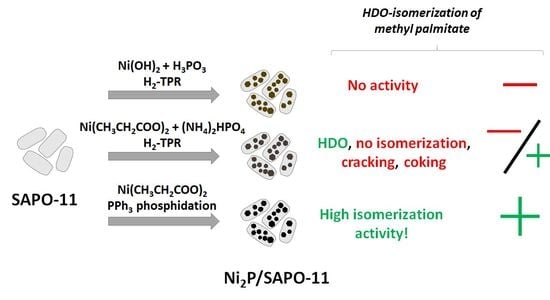
2.1. Catalyst Screening
2.1.1. TPR Catalyst Screening and Search for Optimal Conditions in an Autoclave
2.1.2. Liquid-Phase Phosphidation and Experiments in a Continuous-Flow Reactor
- (1)
- decarbonylation: MP → PA → C16O → C15 hydrocarbons;
- (2)
- direct HDO: MP → PA → C16O → C16OH → C16 hydrocarbons
2.2. Physicochemical Analysis of the TPP Catalysts
2.2.1. Chemical Analysis, Textural Properties, NH3-TPD, and XRD
2.2.2. TEM and SEM Analysis
3. Materials and Methods
3.1. Materials
3.2. SAPO-11 Synthesis
3.3. NiP/SAPO-11 and NiP/SiO2 Synthesis
3.4. Catalyst Characterization
3.5. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Vito Nolfi, G.; Gallucci, K.; Rossi, L. Green Diesel Production by Catalytic Hydrodeoxygenation of Vegetables Oils. Int. J. Environ. Res. Public Health 2021, 18, 13041. [Google Scholar] [CrossRef] [PubMed]
- Yeletsky, P.M.; Kukushkin, R.G.; Yakovlev, V.A.; Chen, B.H. Recent Advances in One-Stage Conversion of Lipid-Based Biomass-Derived Oils into Fuel Components—Aromatics and Isomerized Alkanes. Fuel 2020, 278, 118255. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of Jet Biofuels by Catalytic Hydroprocessing of Esters and Fatty Acids: A Review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Aslam, M.; Kumar, H.; Sarma, A.K.; Kumar, P. Current Status of the Green Diesel Industry. In Green Diesel: An Alternative to Biodiesel and Petrodiesel; Aslam, M., Shivaji Maktedar, S., Sarma, A.K., Eds.; Advances in Sustainability Science and Technology; Springer Nature: Singapore, 2022; pp. 265–283. ISBN 978-981-19223-5-0. [Google Scholar]
- Li, X.; Luo, X.; Jin, Y.; Li, J.; Zhang, H.; Zhang, A.; Xie, J. Heterogeneous Sulfur-Free Hydrodeoxygenation Catalysts for Selectively Upgrading the Renewable Bio-Oils to Second Generation Biofuels. Renew. Sustain. Energy Rev. 2018, 82, 3762–3797. [Google Scholar] [CrossRef]
- Long, F.; Liu, W.; Jiang, X.; Zhai, Q.; Cao, X.; Jiang, J.; Xu, J. State-of-the-Art Technologies for Biofuel Production from Triglycerides: A Review. Renew. Sustain. Energy Rev. 2021, 148, 111269. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Martínez-Klimov, M.; Murzin, D.Y. Hydroconversion of Fatty Acids and Vegetable Oils for Production of Jet Fuels. Fuel 2021, 306, 121673. [Google Scholar] [CrossRef]
- Shamanaeva, I.A.; Yu, Z.; Utemov, A.V.; Wu, W.; Sladkovskiy, D.A.; Parkhomchuk, E.V. Role of Texture and Acidity of SAPO-34 in Methanol to Olefins Conversion. Pet. Chem. 2020, 60, 471–478. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Li, B.; Zhang, J.; Li, Z. A Dual-Bed Strategy for Direct Conversion of Syngas to Light Paraffins. Catalysts 2022, 12, 967. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, Z.; Wang, P.; Liu, X.; Fan, X.; Kong, L.; Xie, Z.; Zhao, Z. Solvent-Free Synthesis of SAPO-34 Zeolite with Tunable SiO2/Al2O3 Ratios for Efficient Catalytic Cracking of 1-Butene. Catalysts 2021, 11, 835. [Google Scholar] [CrossRef]
- Porsin, A.A.; Vlasova, E.N.; Nuzhdin, A.L.; Aleksandrov, P.V.; Bukhtiyarova, G.A. Co-Processing of Straight Run Gas Oil-Rapeseed Oil Mixture Using Sulfide NiMo Catalyst on Zeolite-Containing Support. Russ. J. Appl. Chem. 2019, 92, 1797–1804. [Google Scholar] [CrossRef]
- Pimerzin, A.; Savinov, A.; Vutolkina, A.; Makova, A.; Glotov, A.; Vinokurov, V.; Pimerzin, A. Transition Metal Sulfides- and Noble Metal-Based Catalysts for N-Hexadecane Hydroisomerization: A Study of Poisons Tolerance. Catalysts 2020, 10, 594. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Porsin, A.A.; Aleksandrov, P.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Co-Hydroprocessing of Straight-Run Gasoil—Rapeseed Oil Mixture over Stacked Bed Mo/Al2O3 + NiMo/Al2O3-SAPO-11 Catalysts. Fuel 2021, 285, 119504. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Porsin, A.A.; Aleksandrov, P.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Co-processing of Rapeseed Oil—Straight Run Gas Oil Mixture: Comparative Study of Sulfide CoMo/Al2O3-SAPO-11 and NiMo/Al2O3-SAPO-11 Catalysts. Catal. Today 2021, 378, 119–125. [Google Scholar] [CrossRef]
- Grenev, I.V.; Klimkin, N.D.; Shamanaeva, I.A.; Shubin, A.A.; Chetyrin, I.A.; Gavrilov, V.Y. A Novel Adsorption-Based Method for Revealing the Si Distribution in SAPO Molecular Sieves: The Case of SAPO-11. Microporous Mesoporous Mater. 2021, 328, 111503. [Google Scholar] [CrossRef]
- Freitas, C.; Pereira, M.; Souza, D.; Fonseca, N.; Sales, E.; Frety, R.; Felix, C.; Azevedo, A.; Brandao, S. Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41 Catalysts. Catalysts 2019, 9, 418. [Google Scholar] [CrossRef] [Green Version]
- Auwal, I.A.; Wong, K.-L.; Ling, T.C.; Ooi, B.S.; Ng, E.-P. Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition. Processes 2020, 8, 603. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Lyu, Y.; Liu, Y.; Zhan, W.; Yu, Z.; Fan, L.; Yan, Z. Enhanced Dispersion of Nickel Nanoparticles on SAPO-5 for Boosting Hydroisomerization of n-Hexane. J. Colloid Interface Sci. 2021, 604, 727–736. [Google Scholar] [CrossRef]
- Yang, J.; Kikhtyanin, O.V.; Wu, W.; Zhou, Y.; Toktarev, A.V.; Echevsky, G.V.; Zhang, R. Influence of the Template on the Properties of SAPO-31 and Performance of Pd-Loaded Catalysts for n-Paraffin Isomerization. Microporous Mesoporous Mater. 2012, 150, 14–24. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Kikhtyanin, O.V.; Smirnov, M.Y.; Kalinkin, A.V.; Titkov, A.I.; Ayupov, A.B.; Ermakov, D.Y. Effect of Calcination Temperature on the Properties of Pt/SAPO-31 Catalyst in One-Stage Transformation of Sunflower Oil to Green Diesel. Appl. Catal. A Gen. 2015, 505, 524–531. [Google Scholar] [CrossRef]
- Echevskii, G.V.; Weixin, Q.; Kodenev, E.G.; Toktarev, A.V.; Chunmei, D. Study of Bifunctional Hydrocracking and Hydroisodeparaffinization Catalysts Based on Zeolites and Alumophosphates, Part 2: Effect of the Activity of Hydrogenating and Acidic Components on the Activity and Selectivity of Hydroisodeparaffinization Catalysts. Catal. Ind. 2020, 12, 81–87. [Google Scholar] [CrossRef]
- Wei, X.; Kikhtyanin, O.V.; Parmon, V.N.; Wu, W.; Bai, X.; Zhang, J.; Xiao, L.; Su, X.; Zhang, Y. Synergetic Effect between the Metal and Acid Sites of Pd/SAPO-41 Bifunctional Catalysts in n-Hexadecane Hydroisomerization. J. Porous Mater. 2018, 25, 235–247. [Google Scholar] [CrossRef]
- Phan, D.-P.; Lee, E.Y. Catalytic Hydroisomerization Upgrading of Vegetable Oil-Based Insulating Oil. Catalysts 2018, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Guo, C.; Wang, W.; Bai, X.; Wei, X.; Su, X.; Li, T.; Xiao, L.; Wu, W. The Synergic Effects of Highly Selective Bimetallic Pt-Pd/SAPO-41 Catalysts for the n-Hexadecane Hydroisomerization. Front. Chem. Sci. Eng. 2021, 15, 1111–1124. [Google Scholar] [CrossRef]
- Tiuliukova, I.A.; Rudina, N.A.; Lysikov, A.I.; Cherepanova, S.V.; Parkhomchuk, E.V. Screw-like Morphology of Silicoaluminophosphate-11 (SAPO-11) Crystallized in Ethanol Medium. Mater. Lett. 2018, 228, 61–64. [Google Scholar] [CrossRef]
- Shamanaeva, I.A.; Parkhomchuk, E.V. Variability of Molecular Sieve SAPO-11 Crystals: Acidity, Texture, and Morphology. J. Porous Mater. 2022, 29, 481–492. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, A.K. Recent Developments on Clean Fuels over SAPO-Type Catalysts. In Catalysis for Clean Energy and Environmental Sustainability: Petrochemicals and Refining Processes—Volume 2; Pant, K.K., Gupta, S.K., Ahmad, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 503–525. ISBN 978-3-030-65021-6. [Google Scholar]
- Shamanaeva (Tiuliukova), I.A.; Parkhomchuk, E.V. Influence of the Precursor Preparation Procedure on the Physicochemical Properties of Silicoaluminophosphate SAPO-11. Pet. Chem. 2019, 59, 854–859. [Google Scholar] [CrossRef]
- Ahmad, S.; Ashraf, I.; Mansoor, M.A.; Rizwan, S.; Iqbal, M. An Overview of Recent Advances in the Synthesis and Applications of the Transition Metal Carbide Nanomaterials. Nanomaterials 2021, 11, 776. [Google Scholar] [CrossRef]
- Smith, K.J. Metal Carbides, Phosphides, and Nitrides for Biomass Conversion. Curr. Opin. Green Sustain. Chem. 2020, 22, 47–53. [Google Scholar] [CrossRef]
- Hasanudin, H.; Ryan Asri, W.; Said, M.; Tamara Hidayati, P.; Purwaningrum, W.; Novia, N.; Wijaya, K. Hydrocracking Optimization of Palm Oil to Bio-Gasoline and Bio-Aviation Fuels Using Molybdenum Nitride-Bentonite Catalyst. RSC Adv. 2022, 12, 16431–16443. [Google Scholar] [CrossRef]
- Hasanudin, H.; Ryan Asri, W.; Sari Zulaikha, I.; Ayu, C.; Rachmat, A.; Riyanti, F.; Hadiah, F.; Zainul, R.; Maryana, R. Hydrocracking of Crude Palm Oil to a Biofuel Using Zirconium Nitride and Zirconium Phosphide-Modified Bentonite. RSC Adv. 2022, 12, 21916–21925. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils into Green Diesel. Catalysts 2019, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Golubeva, M.A.; Zakharyan, E.M.; Maximov, A.L. Transition Metal Phosphides (Ni, Co, Mo, W) for Hydrodeoxygenation of Biorefinery Products (a Review). Pet. Chem. 2020, 60, 1109–1128. [Google Scholar] [CrossRef]
- Al-Ali, L.I.; Elmutasim, O.; Al Ali, K.; Singh, N.; Polychronopoulou, K. Transition Metal Phosphides (TMP) as a Versatile Class of Catalysts for the Hydrodeoxygenation Reaction (HDO) of Oil-Derived Compounds. Nanomaterials 2022, 12, 1435. [Google Scholar] [CrossRef]
- Shamanaev, I.; Deliy, I.; Aleksandrov, P.; Gerasimov, E.; Pakharukova, V.; Kodenev, E.; Ayupov, A.; Andreev, A.; Lapina, O.; Bukhtiyarova, G. Effect of Precursor on the Catalytic Properties of Ni2P/SiO2 in Methyl Palmitate Hydrodeoxygenation. RSC Adv. 2016, 6, 30372–30383. [Google Scholar] [CrossRef]
- Deliy, I.V.; Shamanaev, I.V.; Aleksandrov, P.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Yakovlev, I.V.; Lapina, O.B.; Bukhtiyarova, G.A. Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate. Catalysts 2018, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Shamanaev, I.V.; Deliy, I.V.; Aleksandrov, P.V.; Reshetnikov, S.I.; Bukhtiyarova, G.A. Methyl Palmitate Hydrodeoxygenation over Silica-Supported Nickel Phosphide Catalysts in Flow Reactor: Experimental and Kinetic Study. J. Chem. Technol. Biotechnol. 2019, 94, 3007–3019. [Google Scholar] [CrossRef]
- Kaewtrakulchai, N.; Smuthkochorn, A.; Manatura, K.; Panomsuwan, G.; Fuji, M.; Eiad-Ua, A. Porous Biochar Supported Transition Metal Phosphide Catalysts for Hydrocracking of Palm Oil to Bio-Jet Fuel. Materials 2022, 15, 6584. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Yang, Y.; Tian, S. Catalytic Deoxygenation of Methyl Laurate as a Model Compound to Hydrocarbons on Nickel Phosphide Catalysts: Remarkable Support Effect. Fuel Process. Technol. 2014, 118, 161–170. [Google Scholar] [CrossRef]
- Zhao, S.; Li, M.; Chu, Y.; Chen, J. Hydroconversion of Methyl Laurate as a Model Compound to Hydrocarbons on Bifunctional Ni2P/SAPO-11: Simultaneous Comparison with the Performance of Ni/SAPO-11. Energy Fuels 2014, 28, 7122–7132. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Jing, Z.; Xi, K.; Qiao, C. Hydrodeoxygenation of Fatty Acid Methyl Esters and Isomerization of Products over NiP/SAPO-11 Catalysts. J. Fuel Chem. Technol. 2016, 44, 1211–1216. [Google Scholar] [CrossRef]
- Tian, S.; Chen, J. Hydroisomerization of N-Dodecane on a New Kind of Bifunctional Catalyst: Nickel Phosphide Supported on SAPO-11 Molecular Sieve. Fuel Process. Technol. 2014, 122, 120–128. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Q.; Guan, Q.; He, L.; Li, W. Bio-Aviation Fuel Production from Hydroprocessing Castor Oil Promoted by the Nickel-Based Bifunctional Catalysts. Bioresour. Technol. 2015, 183, 93–100. [Google Scholar] [CrossRef]
- Tang, H.; Lin, J.; Cao, Y.; Jibran, K.; Li, J. Influence of NiMoP Phase on Hydrodeoxygenation Pathways of Jatropha Oil. Energy 2022, 243, 123048. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Jiang, X.; Su, X.; Kikhtyanin, O.V.; Wu, W. Hydroisomerization of N-Hexadecane over a Pd–Ni2P/SAPO-31 Bifunctional Catalyst: Synergistic Effects of Bimetallic Active Sites. Catal. Sci. Technol. 2018, 8, 817–828. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Chen, G.; He, Z. Highly Loaded and Dispersed Ni2P/Al2O3 Catalyst with High Selectivity for Hydrogenation of Acetophenone. Catalysts 2018, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Shamanaev, I.V.; Deliy, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Aleksandrov, P.V.; Bukhtiyarova, G.A. Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate. Catalysts 2017, 7, 329. [Google Scholar] [CrossRef] [Green Version]
- Shamanaev, I.V.; Suvorova, A.O.; Gerasimov, E.Y.; Pakharukova, V.P.; Panafidin, M.A.; Yakovlev, I.V.; Bukhtiyarova, G.A. SRGO Hydrotreating over Ni-Phosphide Catalysts on Granulated Al2O3. Catal. Today 2021, 378, 24–32. [Google Scholar] [CrossRef]
- Wang, Y.; Nuzhdin, A.L.; Shamanaev, I.V.; Kodenev, E.G.; Gerasimov, E.Y.; Bukhtiyarova, M.V.; Bukhtiyarova, G.A. Effect of Phosphorus Precursor, Reduction Temperature, and Support on the Catalytic Properties of Nickel Phosphide Catalysts in Continuous-Flow Reductive Amination of Ethyl Levulinate. Int. J. Mol. Sci. 2022, 23, 1106. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Andini, L.; Riyanti, F.; Mara, A.; Hadiah, F.; Fanani, Z. Enhanced Isopropyl Alcohol Conversion over Acidic Nickel Phosphate-Supported Zeolite Catalysts. ACS Omega 2022, 7, 38923–38932. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Li, X.; Prins, R.; Liu, C.; Hao, Q.; Chen, S. Understanding the Reduction of Transition-Metal Phosphates to Transition-Metal Phosphides by Combining Temperature-Programmed Reduction and Infrared Spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 11180–11183. [Google Scholar] [CrossRef] [PubMed]
- Shamanaev, I.V.; Deliy, I.V.; Pakharukova, V.P.; Gerasimov, E.Y.; Rogov, V.A.; Bukhtiyarova, G.A. Effect of the Preparation Conditions on the Physicochemical and Catalytic Properties of Ni2P/SiO2 Catalysts. Russ. Chem. Bull. 2015, 64, 2361–2370. [Google Scholar] [CrossRef]
- Shin, M.; Kim, J.; Suh, Y.-W. Etherification of Biomass-Derived Furanyl Alcohols with Aliphatic Alcohols over Silica-Supported Nickel Phosphide Catalysts: Effect of Surplus P Species on the Acidity. Appl. Catal. A Gen. 2020, 603, 117763. [Google Scholar] [CrossRef]
- Wang, Y.; Nuzhdin, A.L.; Shamanaev, I.V.; Bukhtiyarova, G.A. Flow Synthesis of N-Alkyl-5-Methyl-2-Pyrrolidones over Ni2P/SiO2 Catalyst. Mol. Catal. 2021, 515, 111884. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Li, L.; Li, K. Deoxygenation of Methyl Laurate as a Model Compound to Hydrocarbons on Transition Metal Phosphide Catalysts. Appl. Catal. B Environ. 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Chen, J.; Han, M.; Zhao, S.; Pan, Z.; Zhang, Z. An in Situ Approach to Preparing Ni2P/SiO2 Catalyst under Mild Conditions and Its Performance for the Deoxygenation of Methyl Laurate to Hydrocarbons. Catal. Sci. Technol. 2016, 6, 3938–3949. [Google Scholar] [CrossRef]
- Park, K.-C.; Ihm, S.-K. Comparison of Pt/Zeolite Catalysts for n-Hexadecane Hydroisomerization. Appl. Catal. A Gen. 2000, 203, 201–209. [Google Scholar] [CrossRef]













| Ni/P Ratio | C16/C15 Molar Ratio | Selectivity to n-C15, % | Selectivity to n-C16, % | Yield of n-C15, % | Yield of n-C16, % |
|---|---|---|---|---|---|
| 1/2 | 0.365 | 66 | 24 | 32 | 12 |
| 1/1 | 0.168 | 76 | 13 | 34 | 6 |
| 2/1 | 0.137 | 60 | 8 | 27 | 4 |
| Ni, wt.% | P, wt.% | Vmicro, cm3/g | Vmeso, cm3/g | Smicro, m2/g | SBET, m2/g | NH3-TPD, μmol/g | XRD Phase | DXRD, nm | DTEM, nm | |
|---|---|---|---|---|---|---|---|---|---|---|
| SAPO-11 | – | 24.1 | 0.064 | 0.08 | 166 | 188 | 161 | – | – | – |
| 3% NiP_P | 3.4 | 21.3 | 0 | 0.12 | 0 | 19.5 | 122 | Ni2P | 30 | 6.5 |
| 7% NiP_P | 7.3 | 20.9 | 0 | 0.10 | 0 | 16.0 | 110 | Ni2P | 59 | 9.2 |
| 12% NiP_P | 12.0 | 20.3 | 0 | 0.10 | 0 | 17.6 | 145 | Ni2P | 39 | 9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamanaev, I.V.; Shamanaeva, I.A.; Parkhomchuk, E.V.; Bukhtiyarova, G.A. Hydrodeoxygenation–Isomerization of Methyl Palmitate over SAPO-11-Supported Ni-Phosphide Catalysts. Catalysts 2022, 12, 1486. https://doi.org/10.3390/catal12111486
Shamanaev IV, Shamanaeva IA, Parkhomchuk EV, Bukhtiyarova GA. Hydrodeoxygenation–Isomerization of Methyl Palmitate over SAPO-11-Supported Ni-Phosphide Catalysts. Catalysts. 2022; 12(11):1486. https://doi.org/10.3390/catal12111486
Chicago/Turabian StyleShamanaev, Ivan V., Irina A. Shamanaeva, Ekaterina V. Parkhomchuk, and Galina A. Bukhtiyarova. 2022. "Hydrodeoxygenation–Isomerization of Methyl Palmitate over SAPO-11-Supported Ni-Phosphide Catalysts" Catalysts 12, no. 11: 1486. https://doi.org/10.3390/catal12111486